What's New in 20R3
Pre-Release Date: November 16, 2020 | Release Date: November 20, December 4 & December 18 (CDB), 2020We are pleased to bring you Veeva Clinical Data in 20R3. Read about the new features below. You can find information on enabling new features in the 20R3 Feature Enablement Details. Information on developer features (REST API) is in the Developer Portal.
Clinical Data
Features in this section are changes that apply to all application areas of Veeva EDC and CDB.
Local Labs
Use Case
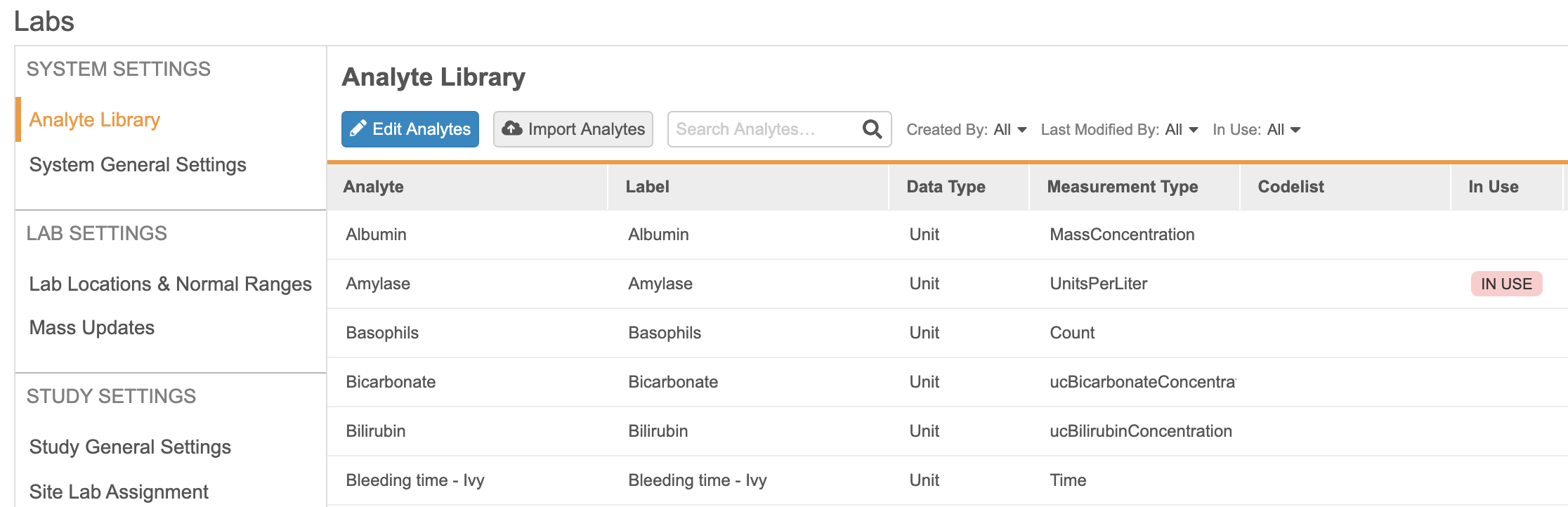
The new Local Labs module provides a way to centralize the management of analytes, lab locations, and normal ranges. It also provides an easy way of configuring lab panels in Studio and simplifies data entry by providing an easy-to-use interface and automatically populating normal ranges when a lab location is selected.
Description
The new Local Labs module allows Lab Data Managers to configure an Analyte Library using lab units and codelists similar to those used in Studio. Lab Data Managers will also be able to maintain a list of lab locations and assign them to the Study Sites that may be using them.
For each Lab Location, Lab Data Managers will be able to manually create normal ranges for any analyte present in the Analyte Library, which includes criteria such as age, sex, and female cycle. These normal ranges will be automatically retrieved when a site selects a lab location in a lab form. If multiple entries need to be created at once, users can utilize the bulk import feature for both lab locations and normal ranges.
Using the Labs module, Lab Data Managers and Study Designers will be able to configure some study-level parameters, such as enabling site override for normal ranges or enabling the use of clinical significance. Lab Data Managers will be able to generate a report detailing the discrepancies between existing data and the updated normal ranges so as to avoid mistakes in the value of normal ranges and determine whether or not those ranges have already been used. Once these differences have been identified, they will have the ability to run a “Mass update” job to automatically update the values present in the Data Entry forms.
In Studio, Study Designers will be able to configure studies to use Local Labs by turning on the “Enable Labs” study setting. When enabled, Study Designers will be able to create lab panels and add them to forms by selecting analytes from the Analyte Library. Study Designers will also have to ensure that specific Casebook Variables are collected in the Schedule, so that the system can automatically retrieve applicable normal ranges for a subject.
When a study is configured to use Local Labs, site users will be asked to enter a lab collection datetime and a lab location in each lab form. The age of the subject can be entered manually or will be calculated by the system. Based on those parameters, the system will then automatically retrieve the normal ranges applicable for the subject. Once the lab results have been entered, the values will be automatically compared to the normal ranges and an indicator will show if any values are out of range. If configured, site users will also be able to enter a clinical significance value or override normal ranges if the ranges retrieved are incorrect.
Enablement & Configuration
All Vaults will be “Labs enabled” after 20R3 and any new study can choose to use Local Labs by turning on the study setting in Studio. Before turning the feature on for a study, users will have to configure several parameters in the Labs tab to set their Vault-level default values.
Randomization
Use Case
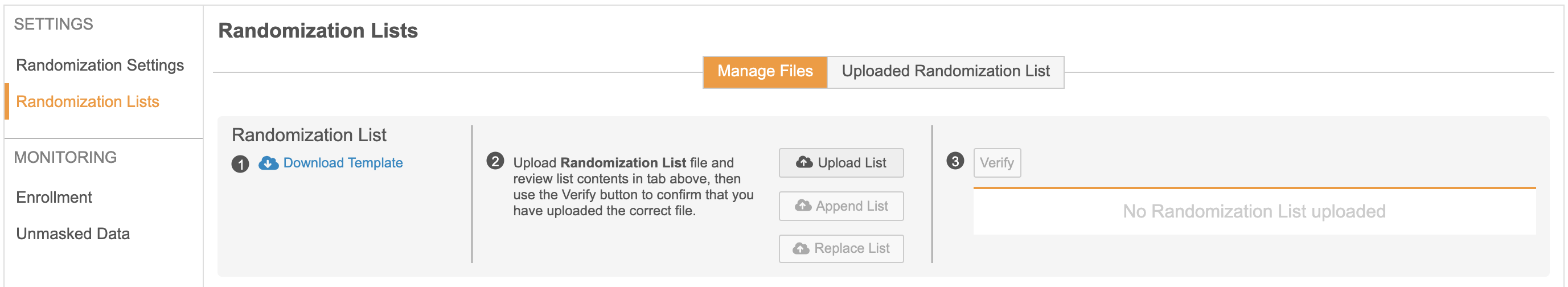
The Veeva Randomization module provides access to simple randomization for studies that do not require the use of a full IRT system. It provides a quick and easy way to configure a randomization strategy, upload a randomization list, and start randomizing subjects.
Description
With this release, Vault CDMS includes a new module for supporting Subject Randomization within a Study. Randomization Managers can choose to configure a study with Simple, Stratified, Stratified with Blocks, or Block randomization. Strata Group configuration, Randomization Lists, Related Data (kits and devices), and additional Randomization settings can all be managed within this new module. As part of the Randomization settings, Randomization Managers can choose to mask – or hide – treatment, kit, and/or device information from Site users. As the study is progressing, Randomization Managers can utilize the Randomization module to monitor enrollment and view kit or device lists.
Within Studio, study designers can enable Randomization by turning on the “Enable Veeva Randomization” study setting. Once enabled, study designers have the ability to configure a custom rule expression to determine the criteria for when a Site user can randomize a Subject. This configuration is available via a new “Ready to Randomize” rule action. Once a rule has been evaluated as “true,”, Subject randomization will be enabled for Site users in the Data Entry tab. Rules can also be evaluated directly after a subject has been randomized to generate the correct schedule based on the treatment assigned.
Once a study is configured in the Randomization module and Studio, Site users will have the ability to randomize a Subject when the preconfigured criteria is met. Once the “Ready to Randomize” rule evaluates as “true” after form submission, Site users will be given the option to randomize the Subject. If the Site user chooses to wait, a “Randomize Now” button will remain available in the Casebook header. Once randomized, the Subject’s randomization ID and related information can be found in the header. Depending on the study settings, Site users can also manually add a kit and/or device ID for randomized Subjects.
Only users with the new “Emergency Unmasking” permission will have the ability to unmask masked data by providing a reason and entering their username and password to authenticate the action. This action, along with other randomization actions, will be recorded in the casebook audit trail.
Enablement & Configuration
The new randomization module must be enabled by request. Once enabled, studies that are not yet published (i.e. do not have a casebook version in production) will be able to turn on Veeva randomization in Studio. Studies already live in production will not be able to use the new Randomization module as there are restrictions in place to prevent updates to published studies.
Upgrade a Study from Data Model Version 1 to Version 2
Use Case
This provides the option to take advantage of new functionality and performance improvements for Studies that were created using data model version 1.0 (created prior to the 20R2 release in August 2020).
Description
With this release, studies on data model version 1.0 now have the option to migrate to the new data model version 2.0 introduced in the 20R2 release. Migrating to data model version 2 allows existing studies to take advantage of new functionality and performance improvements released in, or after, 20R2 requiring the new version.
Enablement & Configuration
Contact your Veeva Services representative to discuss upgrading your study.
Data Review
Features in this section are changes to the Review tab, a working area for clinical research associates and data managers, or to review functionality within the Data Entry tab.
Query Team Restrictions
Use Case
This feature provides the ability limit which users can close another user’s query, which can prevent certain queries from getting closed prior to getting seen and acted on by the correct team.
Description
With this release, the ability and inability for a user to close a manual query created by another user can be enforced within a study. For example, Vault can restrict a user with the CDMS Clinical Research Associate study role from closing a query created by a user with the CDMS Data Manager role. Vault groups Study Roles into Teams at the vault level. All vaults have a set of standard teams to choose from when assigning custom roles to teams. Standard roles are automatically placed into their corresponding teams, but any custom roles must be assigned to a team as a sponsor sees fit. A team can contain multiple roles, but each role can only belong to one team.
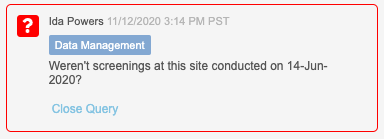
At the study level, study designers can choose to enforce query team restrictions using the Enable Query Team Restrictions setting in Studio > Study Settings. When enabled, the study enforces the restrictions on query closing, based on the vault’s teams. Users will see a team badge on each query, indicating which team created the manual query. The Close Query button is disabled for users who are not in the same team as the user who created the query. Users from any team who have permission to comment on queries can still do so, regardless of the creator’s team.
Additionally, we added a new permission to override query team restrictions, Close All Queries, which controls the ability to close a query regardless of the team that created it. By default, this permission is assigned to the CDMS Lead Data Manager standard study role.
Enablement & Configuration
This feature is available for enablement in vaults where Role by Study is enabled. Query Team Restrictions can only be enabled on studies that have not been previously published.
Veeva Coder
The following are new features for the Veeva Coder application, the Veeva Clinical Data solution for clinical coding.
"How to Code" Coding Configurations Moved to Coder Tools from Studio
Use Case
Because Dictionary Releases are no longer controlled in Studio, there is more flexibility for deployments after upversioning and in changing the dictionary type for a form after coding begins.
Description
While Studio will continue to be the location where we define “what to code”, it’s no longer where we will define “how to code”. All coding method configuration now resides in Coder Tools. In Studio, study designers still identify the Verbatim Item and any Related Items, but coding administrators must define the Dictionary Release, Synonym List, Do Not Autocode List, and Coding Method for each Form in Coder Tools.
Coding on a Form is unavailable until a coding administrator completes those configurations in Coder Tools. This applies to all study environments, including UAT, training, and production, as well as in customer preview (pre-release) environments.
As part of this feature, we renamed the Application Settings tab in Coder Tools as Default Study Settings.
Enablement & Configuration
These changes automatically apply in vaults that contain the Vault Coder application.
Batch Upversioning
Use Case
Coding Administrators will save a lot of time when they upversion their studies once or twice a year, depending on their schedule.
Description
Prior to this release, upversioning applied to a single Form at a time and one Synonym List at a time. With Batch Upversioning, all Forms bound to the same Synonym List can be upversioned at once with a few clicks. Coding administrators have the option to select a subset of Forms and to not include the Synonym List in their batch upversioning action.
So that they may have flexibility in this experience, coding administrators can still upversion a single Form or Synonym List.
Enablement & Configuration
This feature is automatically available in vaults that contain the Vault Coder application, with no additional configuration required.
Third Party Coding Support
Use Case
This feature allows an organization to code EDC data in a clinical coding system other than Vault Coder.
Description
Vault CDMS now supports third-party coding systems for the clinical coding of EDC data. This is only available for new Studies (studies that don’t have any coding requests) and requires the use of the EDC API to manage Code Requests and coding queries.
If a Study is using a third-party coding system, a coder or coding administrator can still navigate to the Coder tab to see a listing of all Code Requests for a Form. There, they can view the audit trail, assigned codes if present, and the query history for each request. They can’t perform any coding or take action on coding queries.
Learn more about the changes to the EDC API in support of this feature on the CDMS Developer Portal.
Enablement & Configuration
The ability to integrate with a third-party coding system, and the ability configure a Study to use that integration_, is available automatically, but an organization must have development support to build an integration between Vault EDC and their coding system.
Simple Autocoding Attribution
Use Case
This change applies automatically in vaults that contain the Vault Coder application, but users must modify reports and dashboards to view this information outside of Admin > Business Admin.
Description
The Code Request object now stores the autocoding source, which can be either the Dictionary or the Synonym List. If the request was autocoded from the synonym list, then the Code Request record stores the name of the Synonym List and the reference to the Synonym. Users can view this information in any report that includes coding data by adding columns for Autocoding Source, Autocoding Synonym List, and Autocoding Synonym List Record. Users can also add dashboard components that compare the efficiency of autocoding from the dictionary to the efficiency of autocoding from the synonym list.
Enablement & Configuration
These changes automatically apply in vaults that contain the Vault Coder application.
Subject Search for Coder
Use Case
This feature provides a quick and easy way to filter Code Requests for one or more Subjects.
Description
The Code Request Listing now includes columns and filters for Site and Subject. This allows coders to search for a specific Subject or set of Subjects to apply coding for the first time or to reevaluate coding. Note that these filters aren’t available when in Group mode.
Enablement & Configuration
This feature is available automatically in vaults that contain the Vault Coder application.
Study Design & Configuration
Features in this area apply to Studio, the study design and configuration area for Veeva EDC.
Time & Events Schedule
Use Case
This feature allows study designers to rapidly build the schedule in a familiar view of the study design.
Description
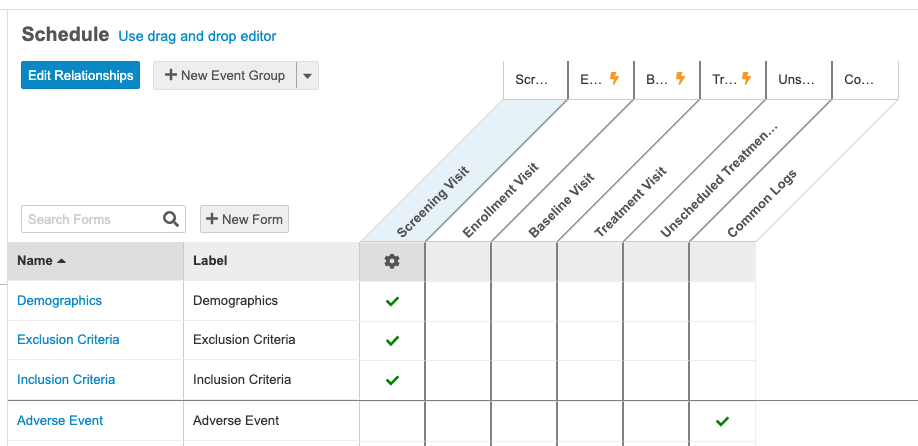
With this release, we enhanced the schedule design environment with a time and events schedule view of the Casebook Definition, similar to the time and events view in the protocol and Study Design Specification. This new view allows users to see and manage their Forms along the left side, and the Event Groups and Events along the top, easily setting which Forms belong with which Events in the resulting grid view.
Users can perform all standard schedule design functions (reordering, adding new or existing Event Groups, Events, and Forms). They can now also add new dynamic rules for an Event Group, Event, or Form by clicking the newly added New Rule button in the Properties panel, which opens the Rule Editor with the appropriate rule action and identifier already selected.
Users can easily switch between the new schedule and the original drag and drop viewer for flexibility and familiarity.
Enablement & Configuration
This feature is only available in Studies that are using version 2 of the expression grammar. To enable the time and events schedule, a Vault Owner must create a new record in the Study Settings object, with the name “schedule_editor_enabled”, the Study, and the value set to “true”.
Study Administration
Features in this section apply to System Tools or EDC Tools, a study-level administration area for Veeva EDC.
Review Plan Assignment Enhancements
Use Case
This feature allows lead data managers to adjust review plan assignment criteria if an existing one no longer is valid while allowing the existing criteria to be unaffected.
Description
With this release, Vault allows deletion of a Review Plan Assignment Criteria if it’s used for assigning a plan to a casebook.
Enablement & Configuration
This change applies automatically in all vaults.
Rule Execution Enhancements
Use Case
This feature allows lead data managers to run a rule that may not have fired. They can also run rules of types other than Query Rules after performing a retrospective amendment.
Description
Lead Data Managers can now view all Rules (except for Set Item Value and Send Email rules) in their study from EDC Tools > Rules (formerly EDC Tools > Query Rules). Users can click on each rule to view additional details, including the rule’s criteria expression. They can use the Advanced Filters to narrow their list of rules, as well as provide a date range for Last Modified Date. They can execute rules on all Subjects or select individual subject casebooks in which to execute rules. There is a preview optional available, which allows users to perform a dry run of their rule execution. Vault doesn’t include locked Casebooks in preview or in rule execution.
Note that users may only select 50 Rules and 500 Casebooks during execution.
Enablement & Configuration
This change applies automatically in all vaults.
Set the Form Status for Impacted Forms during Retrospective Amendments
Use Case
With this feature, it is no longer required to submit a Form or mark it as intentionally left blank if the change results in the form no longer requiring data collection.
Description
Users can now select a Form Status when initiating a retrospective amendment. They can choose to set the impacted Forms in the In Edit or Submitted statuses.
Enablement & Configuration
This change applies automatically in all vaults, and users will see the new option the next time they initiate a retrospective amendment.
Audit Trail Export by Study & Subject
Use Case
Lead data managers may now export the audit history of select subjects, instead of the entire Study.
Description
With this release, we replaced the Audit Trail Export job with Audit Trail Export by Study. Then, we added the Audit Trail Export by Subject job, which allows a lead data manager to select Subjects, including deleted Subjects, to include in the export.
Enablement & Configuration
This feature is automatically available.
Study Administration Functional Enhancements
Description
With this release, we made the following functional enhancements:
- Site Name is no longer required to be unique within the Study.
- We added hover text for the Delete Study Data and Delete Site Data job in EDC Tools > Job History.
- System Tools > External Connections now only displays the Absorb connection, and not the vault connections required for automatic deployments.
Enablement & Configuration
These changes apply automatically in all vaults.
Role Management & Security
Features in this section are enhancements to the System Tools > Role Management and System Tools > Users areas, as well as changes to standard Study Roles, security, and access control in Veeva Clinical Data.
User Management Enhancements
Description
With this release, we made the following changes to enhance the user management experience:
- Added the new API Access permission to control the ability to access the API. This permission is automatically assigned to the CDMS Data Manager and CDMS Lead Data Manager study roles.
- All study roles that are assigned the Manage Study Site permission are now automatically assigned the Manage Study Countries permission.
- Added a new Study Role column to the user listing in EDC Tools > Users, which lists the Study Role assigned to the User.
- Added the ability to show All Roles or only Active Roles in the Role Management table.

Enablement & Configuration
These changes apply automatically in all vaults where Role by Study is enabled.
Deployments
Features in this section are enhancements to deployment functionality in Veeva Clinical Data.
Manage the Deployment List for Custom Roles, Object Tabs Reports & Dashboards from System Tools
Use Case
The ability to add custom roles, reports, and dashboards to the deployment safelist from System Tools, without needing to access Business Admin, increases customer independence.
Description
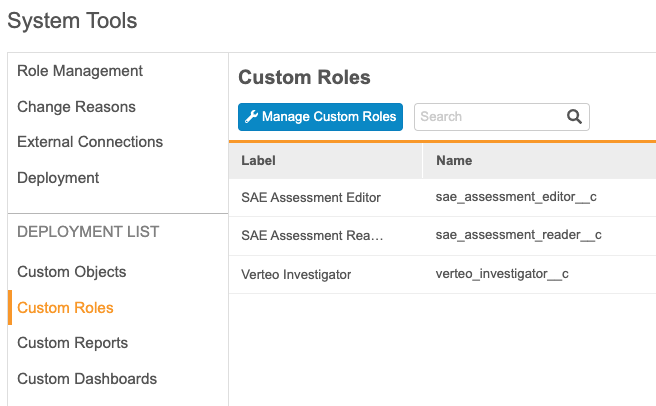
With this release, deployment administrators can now add custom roles, object tabs, reports, and dashboards to the deployment list from within System Tools, without needing to access Business Admin. Deployments can now include custom object tabs, so users no longer need to configure these in every vault. Vault adds custom object tabs to the deployment list when the tab’s object is added to the deployment list.
This feature adds a new section to the System Tools navigation panel, Deployment List, with links to Custom Objects, Custom Roles, Custom Reports, and Custom Dashboards.
Enablement & Configuration
This change applies automatically in all vaults. Vault displays any existing Deployment Whitelist records for these components right away, and a deployment administrator can begin adding additional records immediately.
Status for After-deploy Job
Use Case
This enhancement allows users to easily identify when a deployment is complete but Vault hasn’t yet generated or failed to generate the SDS and comparison report.
Description
We added new statuses to the deployment job, Import Completed and Completed with Documentation Errors. Prior to this release, the Completed status indicated that the import was complete in the target environment, but if there were any errors during SDS and comparison report generation, Vault didn’t indicate that in the job status. Now, the deployment enters the Import Completed status once import is complete, and it only enters the Completed status upon successful generation of the SDS and comparison report. If the documentation generation fails, the deployment enters the Completed with Documentation Errors status, instead of Completed.
Enablement & Configuration
This change applies automatically, and deployment administrators can see these new statuses during their next deployment.
Connections & Integrations
Features in this section are new connections or integrations with Veeva Clinical Data or enhancements to existing ones.
Veeva Learning Integration Enhancements
Use Case
Site users can in real time see the training status for a study and get study access sooner than usual. Lead data managers can more easily manage their role to curriculum mappings.
Description
Site users who haven’t completed their assigned training can now view the training status and navigate to Absorb from the Data Entry tab. Also, users will now see all the studies they have access to.
Lead data managers can now set up learning systems in new studies more easily by copying a Role to Curriculum mapping from another Study in their vault. By default, the curriculum list is now sorted in ascending order. Lead data managers can now delete an existing Learning System from EDC Tools > Learning Systems.
Enablement & Configuration
This change applies automatically in all studies where the Veeva Learning Integration is enabled.
Send SDV Requiredness to CTMS
Use Case
This enhancement provides CTMS users with insight into required monitoring Events and their monitoring outcome.
Description
With this release, CDMS now sends SDV Requiredness for events to CTMS, in addition to the completion status and date. If at least one Review State for an Event is set to Required, SDV is required for the Event. Otherwise, CDMS will send the value, “Not Applicable”.
Enablement & Configuration
This change applies automatically in Studies using the CDMS to CTMS Spark Connection.
Clinical DataBase (CDB) & EDC Clinical Reporting
The following are new features for the Veeva CDB application, EDC Clinical Reporting (the Veeva Clinical Data solution for data cleaning and reporting), or both.
Availability: Clinical DataBase (CDB) is only available to CDB license holders. Contact your Veeva Services representative for details.
Raw Export Type
Use Case
This feature allows the extraction of data from CDB in a raw format that other systems can easily ingest.
Description
With this release, users can create Raw-type Export Definitions, which exports listings and System Listings, which include only contextual header information, in a raw format.
Enablement & Configuration
The feature is available automatically, but users must create a new Export Definition using the Raw type.
Review Data from Listings
Use Case
This feature facilitates the review of data from within Workbench. Data Managers can mark records as reviewed based on their business case and continue reviewing new rows as they are added to the listing. They can quickly access these review listings and further filter the listing data based on summarized review information.
Description
Once a listing is Review Enabled, it becomes a Review Listing. In Review Listings, users can mark each row as Reviewed or as having a known issue. Users can filter the datasheet by the review status, any data items intentionally left blank, and if there are any associated queries. If a Form associated with a listing record was modified after the review was complete, users receive a warning that the data may have changed.
As part of this feature, listings are now grouped into Public Listings, Private Listings, Core Listings, and Review Listings.
Enablement & Configuration
The feature is available automatically, but users must create or edit a listing and toggle on Review Enabled in the listing’s properties.
Support Queries as Listings
Use Case
When reviewing queries for a Study, data managers can sort, filter, and arrange queries in a way that facilitates cleaning or extraction of query details.
Description
With this release, users can sort, filter, and select columns for query listings via the CQL Editor or the query data sheet. Unlike data listings, the selection in these listings is row based and displays the query details similarly to the Queries page.
Workbench includes the following core query listings: Outstanding Queries, Closed Queries, and Query Messages.
Enablement & Configuration
The feature is available automatically, but users must create a query listing.
Link to Query in EDC's Review Tab
Use Case
When reviewing EDC queries from a listing, a data manager may want to view the data as it was entered by the site, in order to better understand the context of the data in question.
Description
With this release, users can navigate directly to a query in the EDC Review tab from a Query in a Workbench Listing, using the link in the Cell Details panel.
Enablement & Configuration
The ability to access EDC directly from a query in a listing is available automatically.
Schedule & Deliver Export Packages
Use Case
Users can now automate and schedule the generation of export packages, as well as retrieve packages generated prior to the most recently generated package.
Description
With this release, Workbench users can schedule the recurring generation and delivery of an export package. Users can select a date and time, frequency, format, and delivery location for each Export Definition. Then, users can view all export packages generated for an Export Definition, download those packages, and view any error messages.
Enablement & Configuration
The feature is available automatically, but users must manually schedule exports.
Export Definition Status Enhancements
Use Case
This feature allows data managers to track the status of an export definition’s readiness when facilitating the preparation of study data for FDA submission.
Description
With this release, users can now mark an Export Listing as Ready when the listing’s formatting and transformation is complete. Once all listings within a definition are in the Ready status, Workbench automatically moves the Export Definition from Draft into the Published status. If any listings within a definition are invalid, Workbench moves the Export Definition into the Invalid status. If any listings return to the Draft status, Workbench moves the Export Definition back into the Draft status.
Workbench displays the status of an Export Definition in Export > Definitions, as well as in the header of the Export Definition Details page. Workbench displays the status of Export Listings in each listing’s row on the Export Definition Details page and in the header of the Export Listing Details page.
Enablement & Configuration
The feature is available automatically. Any existing Export Definitions and Export Listings remain in their Draft statuses.
Manifest File for Export Packages
Description
Export packages now include a manifest file (manifest.json) that describes the content of the package. The manifest lists the Study, the Name of the Export Definition, the datetime of export, the number of files, and then for each file includes the filename, the CQL statement for the Export Listing, and the Name, Type, and Source of each data item.
Enablement & Configuration
This feature is available automatically. Export packages generated after the release will contain the manifest file.
Email Notifications for 3rd Party Data Import
Description
With this release, Workbench sends an email notification to the user when the import of a third party import package is complete.
Enablement & Configuration
This change applies automatically. Users will receive an email notification upon their next import of a third party import package.
CDB Support for Data Model Version 2
Description
Workbench now supports Studies using data model version 2.
Enablement & Configuration
Organizations may work with Veeva Services to upgrade their workbench-enabled Studies to version 2 of the data model.
Enhancements to 3rd Party Data Import
Description
With this release, we made the following enhancements to third party data import:
- Import now supports the ordering of Item Groups in the resulting Core Listings.
- Core Listings for third party data now include headers as columns.
- Third party import supports ISO8601 date formats.
Enablement & Configuration
This changes apply automatically.