New Features in 23R2.4
VeevaID Support, Clinical Data Change Extract, and more...
Release Date: October 13, 2023
We are pleased to bring you the following new features in this week's release. See a summary of feature enablement for this release below. Information on developer features (REST API) is in the Developer Portal.
CDMS
Features in this section are changes that apply to all application areas of Vault CDMS.
Enablement Change: Detail PDF Fast Web View
Description
The Detail PDF Fast Web View feature is now automatically enabled. Prior to this release, this feature was enabled by Veeva Support.
Enablement & Configuration
This feature is available automatically.
Data Entry
Features in this section are changes to the Data Entry tab, a working area for investigators and clinical research coordinators to enter study execution data.
ILB Enhancements
Use Case
These enhancements improve consistency and clarity for users marking forms as ILB or resetting forms. They also prevent forms and items from being linked to forms that are blank or that are marked as ILB.
Description
This feature includes the following changes to the Intentionally Left Blank functionality, which apply to standard and lab forms:
- The option to mark a form as Intentionally Left Blank will now be displayed as a button at the top of the form instead of an action in the action menu. In Lab forms, the button will replace the Lab Tests Not Performed checkbox.
- The ILB button will be disabled in cases when ILB is not allowed because the study or site is locked, the form is locked, frozen, or marked for removal, or there are existing item links.
- The task bar will include blank forms
These changes will affect form linking functionality accordingly:
- Forms marked as ILB cannot be included in form to form or item to form linking - marking a form as ILB after it has been linked will remove form links.
- When a linked form is reset, existing form links for that form will be removed.
- The Reset Form option will be disabled if there are existing item links.
Enablement & Configuration
This feature is automatically enabled in Studies using Form Linking V2 and Data Entry V2.
Learn More
Study Administration
Features in this section apply to EDC Tools, a study-level administration area for Vault EDC.
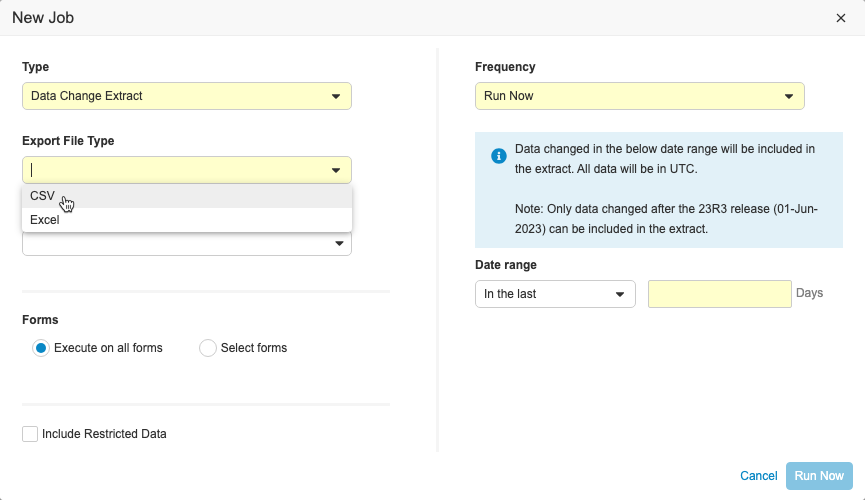
Clinical Data Change Extract
Use Case
This feature provides users with a formatted data extract that is easy to consume when additional monitoring may be required due to frequent data changes.
Description
This feature includes a new job in EDC Tools called the Data Change Extract, where users with the Manage Jobs permission can view changes made to Item and Event Date data during a specific time period. Users can include up to 90 days of data in the extract and specify which forms to include. The extract can be run on ad-hoc or scheduled on a daily, weekly, or monthly basis. The extract is separated into columns so that changes are easily identifiable. Users can also send the extract to an FTP folder.
The Data Change Extract will only include changes to data that occur after the 23R3 release. When testing this feature in pre-release or non-production vaults, Veeva recommends creating a new test study or deleting the old test data and creating new subjects before running the job. If there is existing data and configuration changes are made, the job may not successfully complete.
Enablement & Configuration
This feature is automatically available.
Learn More
Frozen Forms Support for Amendments
Use Case
This enhancement provides more control on how the system manages frozen data when running a retrospective amendment. Data Managers no longer have to manually unfreeze the casebook data in order to apply the amendment. This enhancement is intended for retrospective amendments that include destructive changes.
Description
When performing a retrospective amendment, Vault will automatically retain the frozen status on unimpacted data and will unfreeze data as appropriate where there are changes to the forms. After the retrospective amendment is applied:
- Unit/Codelist Item: when labels change, the Codelist Item is not unfrozen when the change is applied to the frozen Codelist Items
- Item: when Type, Label, Short Label, or Hint Labels are changed, the Item is unfrozen if there is a change to the Item
- Item Group: when Override Label or Short Label are changed, the Item Group is unfrozen if there is a change to the Item Group
- Form: when Form properties change (e.g., form label or description), the Form is not unfrozen when the change is applied to the frozen Form
- Event: when a form is added or removed from an Event, the Event is not unfrozen when the change applies to the frozen Event
- Casebooks: when a Casebook is frozen and there is a change to the subject, the Casebook is not unfrozen when the change applies to the frozen Casebook
- New Items, Item Groups, Forms, and Events added will be added in an unfrozen state (New Item Groups added will have all of the items added in an unfrozen state)
Enablement & Configuration
Contact Veeva Support to enable this feature in your vault. This is an update for studies operating in Data Model 2. Studies in Data Model 1 had already operated in this manner and will remain the same, as described above for retrospective amendments. Note that Veeva will only enable this feature when there is a retrospective amendment with destructive changes.
Role Management & Security
Features in this section are enhancements to the System Tools > Role Management and System Tools > Users areas, as well as changes to standard Study Roles, security, and access control in Vault CDMS.
VeevaID Support
Use Case
This feature provides support for linking existing VeevaID users with new or updated CDMS users. It primarily benefits site users who already have a VeevaID to reduce the number of separate logins the user has for different sponsor’s studies and Veeva products. This feature also supports user administrators with managing existing accounts.
Description
When adding new Users to a vault, Vault can now check the user’s email to confirm if the account belongs to an existing user with a VeevaID. When the user already has a valid VeevaID, Vault adds the user to the vault as a cross-domain user with their VeevaID security policy.
Once a VeevaID user is created, they are a cross-domain user, and so you can only make changes to User Name, Study Role, Site Access, and Country Access.
In support of this feature, we added a new, optional column to the user import template, Bypass VeevaID Check. By default, this is set to “No”. When this column is set to “Yes”, Vault skips the VeevaID verification and instead creates the user strictly based on the provided User Name. This is useful when a user needs separate accounts for user acceptance testing where VeevaID may not be desired.
Enablement & Configuration
Contact Veeva Support to discuss this feature.
Integrations
Features in this section are new integrations with Vault CDMS or enhancements to existing integrations.
Safety Configuration Moved to Studio
Use Case
Hosting the Safety Integration configurations in Studio will allow the application of the EDC library and deployment capabilities onto the Safety Integration. The new Form Configuration wizard supports the Safety to EDC form item mappings, but also provides enhanced options for multiple mappings for each Safety Form Type as well as new Inclusion Criteria for Study Drugs into a safety case.
Description
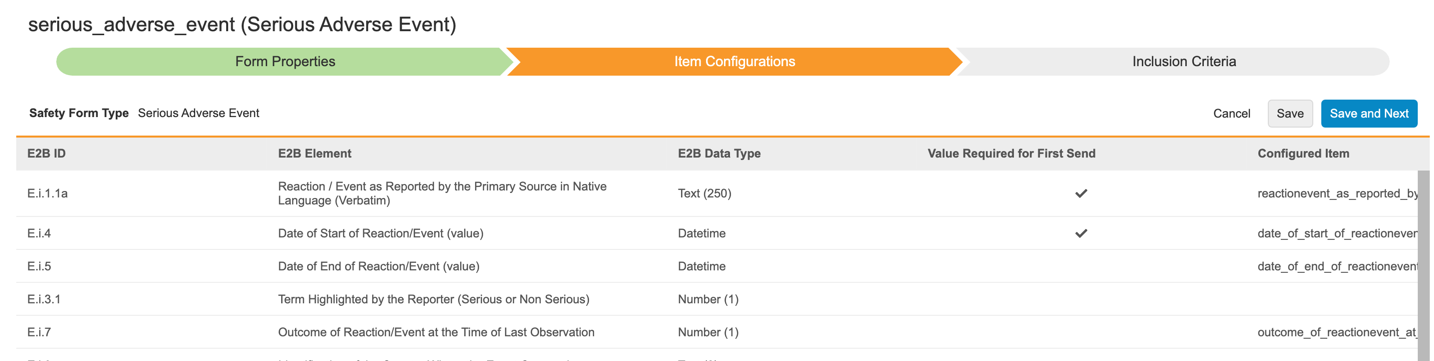
With this release, we moved all Safety Integration configurations from EDC Tools to Studio.
Study settings are now extended by Safety Integration Type. The available types are E2B - Vault Safety, for integration with the Vault Safety application, and E2B - External for external safety systems.
Users can configure their study-specific Safety Settings and Form Configurations from the new Safety Configuration area of Studio. Form Configurations are now performed in a wizard, which guides users through the mapping of safety form types to EDC forms and their respective items, and thereafter the selection of inclusion criteria into the safety case.
While the Integration Setup and Job Scheduler are shown under the Safety Settings, they are configured in Tools > Safety Integrations > Safety Settings as these settings differ by environment and are not deployed.
As part of this feature, additional capabilities for the CDMS Safety Integrations are now in place:
- The Safety Case Inclusion Criteria for Study Drugs have been enhanced to allow the selection of the first dispense date as well as closest on/before and closest on/after the SAE start date to be included.
- Each Safety Form Type can now be mapped to multiple EDC forms.
- Entries in a Safety Case can now also be from repeating item groups, not just forms across all Safety Form Types
- The driving Safety Key Item of SAE and Study Drug dispense forms can be configured with either a defined value or now alternatively ‘Any non empty value’. This allows e.g. for configuring a dispense form to be a candidate to be in the Safety Case simply by a non-empty Start Date.
- The NullFlavors for coded fields have been amended with the ‘10067482’ code.
Enablement & Configuration
This feature is automatically enabled.
EDC Migrator
Features in this section are new features for Veeva EDC Migrator.
Setting Subject Statuses
Use Case
The system now automates the setting of Subject Statuses so users no longer have to manually run status rules in multiple batches.
Description
With this release, the system will automate the setting of Subject Statuses as part of the Workflow Progress table. The setting of Subject Statuses can be initiated by using the Execute or Execute All Steps feature.
When the Execute All Steps feature is used, Subject Statuses are only set if the previous step is a success. If the previous step fails, the Subject Statuses step does not auto-start. The step is also optional and can be skipped.
In Migration Vault, Subject Status rules are executed in the following order:
- Pre Screen
- Consented
- In Screening
- Screen Failure
- Enrolled
- Randomized
- Started Treatment
- End of Treatment
- Withdrawn
- Started Follow Up
- Lost to Follow Up
- Complete
Enablement & Configuration
This feature is automatically enabled. Users must implement the default CDMS Subject Status execution order.
Skipping a Step during Execute All & Rewording Stop Link
Use Case
The skipping enhancement supports execution automation and reduces manual effort for users who need to skip a workflow step. The rewording of the Stop link improves the user experience by clarifying the actions taken by the user, whether stopping a step or stopping the workflow.
Description
Previously, users could not skip individual Workflow Progress steps when using the Execute All Steps feature. If a specific step needed to be skipped, the feature could not be utilized. Now, users can skip optional steps when using this feature.
The Stop link in the Workflow Progress table now reflects the actions taken by the user. If a user chooses to stop the Execute All Steps workflow, they’ll see the new Stop Step option, which reflects the user’s ability to stop steps individually. Previously, the only option was Stop, which didn’t distinguish between stopping a step and stopping the entire workflow.
Enablement & Configuration
This feature is automatically enabled but users must select the steps they want to skip prior to executing a load.
Feature Enablement Summary
| Feature Name | Configuration | Dependencies | Day 1 Impact to Primary Users | Users with Day 1 Visibility |
|---|---|---|---|---|
| CDMS | ||||
| Data Entry | ||||
| ILB Enhancements | Form Linking V2, Data Entry V2 |
|
|
|
| Study Administration | ||||
| Clinical Data Change Extract |
|
|||
| Frozen Forms Support for Amendments | Support | Data Model V2 |
|
|
| Role Management & Security | ||||
| VeevaID Support | Support | |||
| EDC Migrator | ||||
| Limited Availability: In the current release, EDC Migrator is only available to early adopter customers. Contact your Veeva Services representative for details. | ||||
| Setting Subject Statuses |
|
|||
| Skipping a Step during Execute All & Rewording Stop Link |
|
|||
| Integrations | ||||
| Safety Configuration Moved to Studio | Data Model V2 |
|
||
Enablement Legend
- Configuration: This field lists the location(s) where configuration for this feature occurs, for example, "Studio" or "EDC Tools". "Support" indicates that this feature must be enabled by Veeva Support, and "Vault Admin" indicates that configuration must be performed by a Vault Owner in the vault's Admin area.
- Dependencies: This field lists any dependencies required to use this feature, for example, Labs or Expression Engine V2. The other columns assume that the dependencies are enabled/in use.
- Day 1 Impact to Primary Users: This feature is visible and available to one or more primary user teams (Site Users, Clinical Team, and Coders) on day 1. Otherwise, this feature is either only visible to study designers or administrator users, it requires configuration before it is visible to primary users.
- Users with Day 1 Visibility: This feature is visible to these users on day 1 if no configuration occurs.