New Features in 22R2.4
Cross-Form Derivations, Sign with Open Queries, and more...
Release Date: October 14, 2022
We are pleased to bring you the following new features in this week's release. See a summary of feature enablement for this release below. Information on developer features (REST API) is in the Developer Portal.
Data Entry
Features in this section are changes to the Data Entry tab, a working area for investigators and clinical research coordinators to enter study execution data.
Bulk Casebook Signature
Use Case
Principal Investigators can sign multiple casebooks at once rather than signing each individual casebook at a time.
Description
This feature allows Principal Investigators to sign multiple casebooks at a time for a site. This feature also introduces the Signature History page in Data Entry, where users can view the signature history for all signing activities, both bulk and non-bulk. This page is available regardless of whether bulk casebook signature is enabled in a vault.
Enablement & Configuration
Contact Veeva Support to enable this feature. Once enabled at the Vault level, a Lead Data Manager must enable it for a study from EDC Tools then for individual sites in EDC Tools > Sites.
Show the Delimiter for Composite Items in Data Entry
Use Case
Item labels will now be consistent for composite items when the form is blank or in Edit or View mode.
Description
The / Delimiter will now always be visible in the label for composite items in both Edit and View modes of Data Entry.
Enablement & Configuration
This change applies automatically.
Sign with Open Queries
Use Case
There can be times during the lifecycle of a study when all existing forms and event dates need to be signed regardless of whether or not there are open queries. This feature allows those forms and event dates to be signed when necessary.
Description
Principal Investigators can apply their signatures to forms and event dates that have open queries.
Enablement & Configuration
Contact Veeva Support to enable this feature.
Study Design & Configuration
Features in this area apply to Studio, the study design and configuration area for Vault EDC.
Cross-Form Derivations
Use Case
The traditional challenge that cross-form calculations pose on EDC systems is that changing data on one form that is used to derive values onto another requires manual action from the user to the form with the resulting value. This feature provides a way to carry forward values from one form to another while displaying accurate, updated data even on submitted and non-materialized forms.
Description
With this release, we’ve added the ability to derive values across forms in a casebook. Study Designers can add the new “Derived Items” component to forms and create Set Derived Value rules to set values of derived items based on criteria across a casebook.
Derived items differ from traditional item types in that derived item values don’t depend on the submission of a form, and derived values can be updated outside of the constraints of traditional Data Entry (such as form submission, study locking and freezing, and SDV/DMR). Derived items can be configured to display or not display in both Data Entry and extracts.
EDC Tools users can now run Set Derived Values rules as part of the Run Rules job in Tools > EDC Tools > Rules.
Enablement & Configuration
The Derivation Version must be set to “Cross Form” on the study’s Study Configuration record.
Rules: Referencing the Previous Value of an Object with @PreviousEvent
Use Case
This feature reduces the need for custom triggers. It also provides study designers with more flexibility when designing query rules and reduces the need for CRAs and data managers to perform manual checks on blank data.
Description
Query rules can now locate previous values for Events, Forms, and Items in a Casebook, given the context of the current Form being submitted and its parent Event. The previous Event is determined using the chronological order of Events in the schedule.
Values that occur throughout a Study but don’t necessarily repeat or follow a set pattern often need to be compared to a value in the previous iteration of that Form, or to another value in the previous Event. This feature allows data to be referenced based on the chronological order in which a subject progresses through visits. For example, a rule can now compare a subject’s dosing record at a given Event with their dosing record from the Previous Event. When event dates change, or occur out of chronological order, or when Unscheduled Events are inserted between scheduled study visits, @PreviousEvent rules will identify such changes and be re-evaluated in the context of a casebook’s current event date order.
The identifier @PreviousEvent must be used along with @Form identifiers, which are required to give context to determine the previous value. @PreviousEvent rules are executed as post-save rules.
As part of this enhancement, for rules using the [-1] and [+1] functionality to determine the previous or next value of a repeating object, users can now configure whether the rule should skip over values that have been marked as Intentionally Left Blank.
Enablement & Configuration
This feature is automatically available to Studies using Expression Engine V2.
Multi-Language Support in Studio
Use Case
While the ability to import translations was possible through Vault Platform translation features, this feature simplifies that process by allowing Studio users to import and export their study and version specific Labels for their definitions.
Description
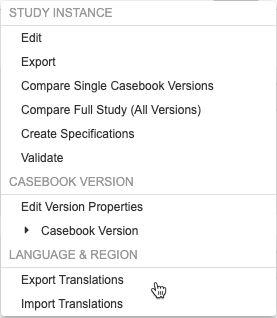
Studio users can now import and export translated study labels and messages for use in a translation workbench. Users can choose one or more languages to export, send those to a translation service, and then import the translations. Studio can process multiple languages at once, and each language goes into its own tab-delimited CSV file. While the file format is CSV, the files are tab delimited, as study labels can contain commas. The Export Translations and Import Translations actions are available in the new Language & Region section of the Studio Actions menu.
Translations must be done as part of the study’s build process and prior to deploying to production.
This release doesn’t include translations for vault-level labels for Lab Analytes or custom Reasons for Change and Intentionally Left Blank Reasons.
Enablement & Configuration
This feature is available automatically in Studio. This functionality was previously only available in Admin > Settings.
Learn More
Form and Item Linking Rules Allow References to Fully-qualified Identifiers
Use Case
This enhancement allows more data to be accessed by a single rule, reducing the need for custom triggers.
Description
With this release, rules containing Form and Item links now allow references to fully-qualified identifiers outside of the linked identifiers. Linking Rules that contain fully-qualified outside identifiers will automatically be required for execution as a post-save rule.
Enablement & Configuration
This feature is automatically available for Studies using Expression Engine V2.
Enablement Change: Option to Include Keys & Origin in SDS
Description
The “Option to Include Keys & Origin in SDS” feature is now automatically enabled. Studio users can choose to include additional key values in their SDS to use for troubleshooting or tracking definitions across other applications, such as a metadata repository.
Enablement & Configuration
This feature is now auto-on. Studies created prior to the 21R1 release (initial release of the Library) may have missing origin records. Studies created after the 21R1 release have all origin records.
Study Administration
Features in this section apply to EDC Tools, a study-level administration area for Vault EDC.
Events Support Event Window
Use Case
With this change, the Event Window Status and Days Out of Window will calculate based on the new Event Window Definition.
Description
The system will now calculate if an event is “Out of Window,” and if so, how many days out of window.
Enablement & Configuration
This feature is available automatically.
Include Restricted Forms and Events in Counts for Subject Progress and Event Progress Listings
Use Case
With this feature, count columns will respect the restricted job option.
Description
If the user selects Include Restricted Data in the Subject Progress Listing or Event Progress Listing job dialog, the job will include restricted forms and events in columns that count the number of forms and events. If “Include Restricted Data” isn’t selected, the listings won’t include restricted forms and events.
Enablement & Configuration
This feature is automatically available.
Include User Role in Query Detail Listings for Studies Not Using Query Teams
Use Case
Users can identify the roles that are creating, answering and closing queries, even if the Query Teams feature is not enabled.
Description
When a study isn’t using the Query Teams feature, Vault now populates the following Query Detail Listing columns with the user’s current study role: Created By Role, Answered By Role, and Closed By Role.
Enablement & Configuration
This feature is automatically available.
Include Site Number in Study Summary Metrics Report
Use Case
Users can now identify a site by its number or its name.
Description
The Study Summary Metrics Report now includes a column for Site Number.
Enablement & Configuration
This feature is automatically available.
Deployments
Features in this section are enhancements to deployment functionality in Vault CDMS.
Retrospective Amendment Start Event Requires the First Event
Use Case
This change is to avoid issues with mixed-version Casebooks (where a Subject has Event Groups with their Events and Forms on different versions).
Description
This feature removes the ability to select the Start Event for a retrospective amendment. Now, Vault automatically uses the first Event in the first Event Group on the casebook schedule. Vault now only permits retrospective amendments on the entire Casebook starting from the first Event Group and Event.
Enablement & Configuration
This change applies automatically to all Studies.
Labs
Features in this section are new features for the Labs module of Vault EDC.
Labs: Support for Lab Results with Characters '>' and '<'
Use Case
This feature provides additional support for lab results that are more or less than numbers or units.
Description
With this feature, users can use “>” and “<” characters when entering Lab results in Data Entry.
Enablement & Configuration
This feature is automatically enabled in Studies using Data Model V2 and Global Versionless Labs.
Labs: Ability to Disable Pending Labs
Use Case
This feature provides flexibility by allowing or not allowing Sites to add new Lab Locations.
Description
With this release, users can configure whether or not Sites can enter new lab locations and associated lab normals.
Enablement & Configuration
This feature is available automatically in Studies using Labs.
Clinical DataBase (CDB)
The following are new features for the CDB application, the Vault CDMS solution for data cleaning and reporting.
Availability: Clinical DataBase (CDB) is only available to CDB license holders. Contact your Veeva Services representative for details.
Reference Objects
Use Case
Reference objects address the need to define non-clinical data during the import process, so that the data can be used alongside clinical data for cleaning and export purposes.
Description
CQL now supports reference objects. Reference objects are like Forms, in that they contain a limited set of Items, but they are different in that a reference object must define how it relates to other header or form data. This relationship is defined upon ingestion. Once the data has been defined and ingested, CQL can use it like any other Form.
Reference objects are defined in the manifest file for an import package. Then, the data for the reference object is imported as a CSV file in that import package.
This feature introduces a new CQL function, KEYMATCH, which is only available for use with reference objects. The KEYMATCH function checks if the related_item matches the related_key.
Enablement & Configuration
The Reference Objects feature is enabled by default, but users must define reference objects to use them.
Automated Checks Re-query
Use Case
Organizations can automate re-queries.
Description
After a check opens a query against a record, and that query is closed, if a data change occurs such that the record reappears in the check, CDB re-queries the Item or Event Date.
Enablement & Configuration
This feature is available automatically. Automated checks begin re-querying automatically, with no additional configuration required.
CDB Data Provider Query Access
Use Case
Organizations can now allow data providers access to a given data source to answer queries.
Description
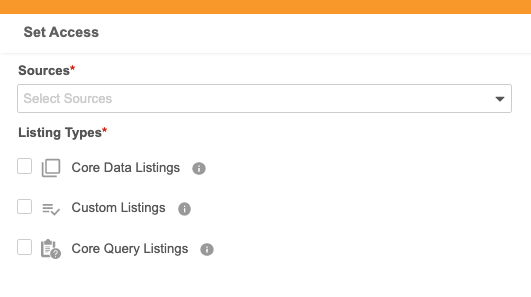
Administrators can now define a user’s source access (for data providers) to answering queries within CDB > Admin > Users. Once given access, data providers can access select listings in CDB to answer queries associated with their specific data source.
This feature adds the following new permissions:
Assigned to the CDB Data Provider, CDMS Super User, and CDMS Data Manager study roles:
- View Selected Listings
- View Selected Query Listings
- Answer 3rd Party Queries
Assigned to the CDMS Super User, CDMS Data Manager, and CDMS Lead Data Manager study roles:
- View All Listings
- View All CDB Query Listings
Enablement & Configuration
This feature is available automatically. Vault Administrators can set user access from Admin > Users.
Learn More
Route 3rd Party Packages to the Right Vault in the Domain
Use Case
This feature allows for easier management of FTP credentials when uploading data to CDB.
Description
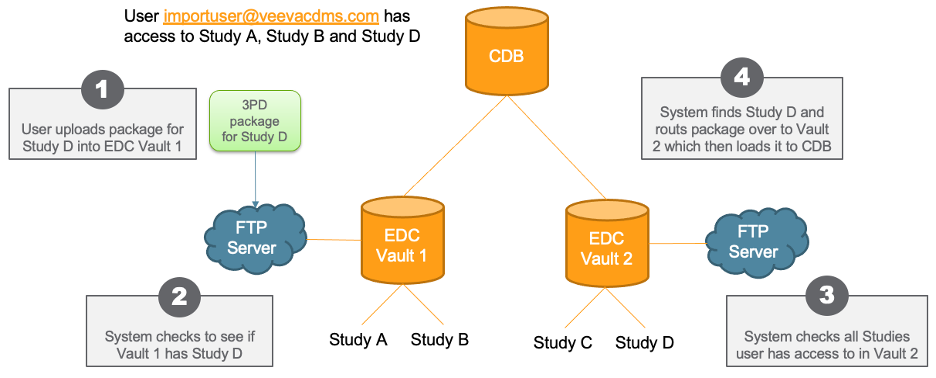
For organizations with more than one vaults in their domain, their credentials are different for each vault because the username contains the the vault’s DNS and the Vault User Name, for example, “prod1-pharmacdms.veevavault.com+tsmith@veepharm.com”. Because a customer or data vendor may not know exactly which vault a Study resides in, they may not know which user name to use when uploading their package to the FTP server.
To minimize confusion, CDB now automatically checks all vaults that a user can access for the Study referenced in their package. If CDB finds the Study in the package in the current vault, then CDB loads the package as it does today. If the current vault doesn’t contain the Study, CDB checks all other vaults the user can access. When it finds the vault containing the Study, CDB loads the package into that vault. Users must be able to access the FTP server in the appropriate vault to load the Study.
Enablement & Configuration
This feature is enabled automatically.
Support for Queries on CDB Derived Fields
Use Case
Users no longer need to access the source Item for a derived field to open or close queries against the data point.
Description
Users can now view the cell details for a CDB univariate, derived field. From the Cell Details panel, they can now open and close queries on the underlying EDC or third party data Item.
Enablement & Configuration
This feature is enabled automatically to all users.
3rd Party Data Load Error Rollback
Use Case
During the course of a study, if a vendor loads a bad package, the study isn’t blocked from ingesting data from other vendors. This ensures that the cleaning of data from other sources can continue and that an errored source won’t result in a bottleneck.
Description
With this release, if the import of a third party data package fails, CDB now automatically rolls back to the last successfully loaded package for that Source. Prior to this release, if a load failed, all new data from any source was blocked until the error was corrected.
If there isn’t a successful package for the source to rollback to, then no new data is imported from any source until the error is fixed.
Enablement & Configuration
This feature is enabled automatically.
Integrations
Features in this section are new integrations with Vault CDMS or enhancements to existing integrations.
E2BLink: Email Notifications
Use Case
Users can receive notifications when a Safety transmission fails and even prior to it failing so that the connection administrator can take action.
Description
E2BLink captures up to five email addresses to send email notifications when the transmission fails via the Safety to CDMS Connector or via the AS2 Gateway to a third party Safety System. It will also send an email prior to the expiration of the AS2 Gateway Encryption Certificates.
Vault can now send email notifications when a transmission fails, there is an expired certificate, or there is an inactive connection or gateway profile. Vault also can send a notification when the AS2 Gateway Encryption Certificate is about to expire. Vault sends the notification three months prior, one month prior, and the day the certificates expire, giving your team enough time to respond and load new certificates. Administrators can specify up to five (5) email addresses to receive these notifications from Tools > System Tools > External Connections.
As part of this feature, we changed some UI labels in EDC Tools > Safety Configuration:
| Original Label | New Label |
|---|---|
| Safety System | Safety System Connection Profile |
| Send Adverse Events to Safety System | Study Transmission Status |
| Treatment | Study Drug |
| Include Treatment Form | Include Study Drug Form |
| Treatment Form | Study Drug Form |
| Treatment Name | Study Drug Name |
| Item Indicating Treatment Administered | Item Indicating Study Drug Administered |
| Item Value Indicating Treatment Administered | Item Value Indicating Study Drug Administered |
| Add Treatment | Add Study Drug |
Enablement & Configuration
This feature is available automatically.
Feature Enablement Summary
| Feature Name | Configuration | Dependencies | Day 1 Impact to Primary Users | Users with Day 1 Visibility |
|---|---|---|---|---|
| Data Entry | ||||
| Bulk Casebook Signature | EDC Tools, Support | Data Model V2 | ||
| Show the Delimiter for Composite Items in Data Entry |
|
|
||
| Sign with Open Queries | EDC Tools, Support | Data Model V2 | ||
| Study Administration | ||||
| Events Support Event Window |
|
|||
| Include Restricted Forms and Events in Counts for Subject Progress and Event Progress Listings | Query Teams disabled |
|
||
| Include User Role in Query Detail Listings for Studies Not Using Query Teams | Query Teams Disabled |
|
||
| Include Site Number in Study Summary Metrics Report |
|
|||
| Study Design & Configuration | ||||
| Cross-Form Derivations | Vault Admin |
|
||
| Rules: Referencing the Previous Value of an Object with @PreviousEvent | Expression Engine V2 |
|
||
| Multi-Language Support in Studio |
|
|||
| Form and Item Linking Rules Allow References to Fully-qualified Identifiers | Expression Engine V2 |
|
||
| Labs | ||||
| Labs: Support for Lab Results with Characters '>' and '<' | Labs | Labs |
|
|
| Labs: Ability to Disable Pending Labs | Labs | Labs |
|
|
| Deployments | ||||
| Retrospective Amendment Start Event Requires the First Event |
|
|||
| Integrations | ||||
| E2BLink: Email Notifications | System Tools |
|
||
| Vault CDB | ||||
| Reference Objects |
|
|||
| Automated Checks Re-query |
|
|||
| CDB Data Provider Query Access |
|
|||
| Route 3rd Party Packages to the Right Vault in the Domain |
|
|||
| Support for Queries on CDB Derived Fields |
|
|||
| 3rd Party Data Load Error Rollback |
|
|||
Enablement Legend
- Configuration: This field lists the location(s) where configuration for this feature occurs, for example, "Studio" or "EDC Tools". "Support" indicates that this feature must be enabled by Veeva Support, and "Vault Admin" indicates that configuration must be performed by a Vault Owner in the vault's Admin area.
- Dependencies: This field lists any dependencies required to use this feature, for example, Labs or Expression Engine V2. The other columns assume that the dependencies are enabled/in use.
- Day 1 Impact to Primary Users: This feature is visible and available to one or more primary user teams (Site Users, Clinical Team, and Coders) on day 1. Otherwise, this feature is either only visible to study designers or administrator users, it requires configuration before it is visible to primary users.
- Users with Day 1 Visibility: This feature is visible to these users on day 1 if no configuration occurs.