New Features in 22R2.3
New SDS Options, Configurable Lab Queries, and more...
Release Date: September 23, 2022
We are pleased to bring you the following new features in this week's release. See a summary of feature enablement for this release below. Information on developer features (REST API) is in the Developer Portal.
Data Entry
Features in this section are changes to the Data Entry tab, a working area for investigators and clinical research coordinators to enter study execution data.
Default Settings for Data Entry
Use Case
There is more room on the screen for data entry, especially for users with smaller window sizes.
Description
The schedule tree in Data Entry is now responsive to the user’s browser window, devoting more space on the screen to data entry. The width of the schedule tree will still be customizable but will take up one-third of the Data Entry screen by default. If the user chooses to customize the schedule tree width, the selected width will stick across logged in sessions. In addition, the default height of the Common Log section is higher than previous releases to provide the user with better visibility.
Enablement & Configuration
This feature is available automatically.
Data Review
Features in this section are changes to the Review tab, a working area for clinical research associates and data managers, or to review functionality within the Data Entry tab.
Ability to Specify Other Reason for Additive Review
Use Case
This feature provides flexibility for users performing Additive Review.
Description
When the “Enable Other Specify” reason for change and “Enable Additive Review” option are both set to “Yes” in Studio, CRAs and Data Managers can provide a custom reason for Additive Review.
Enablement & Configuration
This feature is automatically enabled for Studies where Additive Review is enabled.
Protocol Deviations Enhancements
Description
With this release, we have made various enhancements to Protocol Deviations which are detailed below.
- We have added a new column, Protocol Deviation ID, which will display in the Protocol Deviation grid and in both exports. This column allows users to find a deviation in the export by its unique ID number.
- We have made improvements to the Protocol Deviation search, so that users will now be able to search on the Protocol Deviation ID or Summary. In addition, the search is more flexible and case insensitive, so users no longer need to enter an exact match.
- When viewing a Protocol Deviation in the Review UI, users will now be able to see the deviation’s status in the dialog. Users will only see active Protocol Deviations, such as those with an Open or Investigating status. Inactive Protocol Deviations can only be viewed in the Protocol Deviation grid.
Enablement & Configuration
These enhancements are automatically available in Studies using Protocol Deviations.
Protocol Deviations: Export as an Excel™ File
Description
Protocol Deviations can now be exported as an Excel™ file. The Date fields in both the CSV and Excel™ exports also have been updated to ISO format (YYYY-MM-DD). In addition, users can now export a filtered list of deviations.
Enablement & Configuration
This feature are automatically available in Studies using Protocol Deviations.
Clinical Coding
The following are new features for Coder, the clinical coding area for Vault Coder.
Coder UI Enhancements
Description
We have made the following UI enhancements to Coder with this release:
- Collapsible Properties Panel: users can collapse and expand the Properties Panel as necessary while coding
- Optimized white space: the Verbatims table will now occupy available white space to display more Verbatims
Enablement & Configuration
This feature is enabled automatically.
Coders are Allowed to Code a Form with a "Needs Synonym List" Status
Use Case
This feature allows Coder more freedom to complete their tasks while Coder Administrators are administering Synonym Lists.
Description
If a Form does not have an assigned Synonym List then the Coder may continue to code the Form until one is assigned. Forms with the Needs Synonym List status will not Autocode or have Suggestions off of the Synonym List.
Enablement & Configuration
This change applies automatically.
New Upversioning Status in Coder
Use Case
Users can easily identify which Forms and Synonym Lists failed Upversioning so that they can restart Upversioning on these Forms and Synonym Lists.
Description
With this release, we’ve introduced a new status called Failed Upversioning that will display when Upversioning fails for a Form or Synonym List. This status allows Coder Administrators to easily identify which Forms and Synonym Lists failed Upversioning. The Upversioning job has also been improved so that Coder can upversion more code requests in less time. As part of this change, the code request object no longer maintains the Dictionary Release field. To reference Dictionary Release data in Vault Reports, view the Dictionary Release field value from the Medical Coding Item Definition object.
The Failed Upversioning status is visible to Coders on the Coder home page and Verbatims page. Vault will notify users when a Form has the Failed Upversioning status. Users can continue to code the Form until the Coder Administrator restarts Upversioning.
Synonym Lists in this status cannot not be assigned to any Forms until a Coder Administrator restarts Upversioning and the upversion succeeds.
Enablement & Configuration
Auto-on.
Study Design & Configuration
Features in this area apply to Studio, the study design and configuration area for Vault EDC.
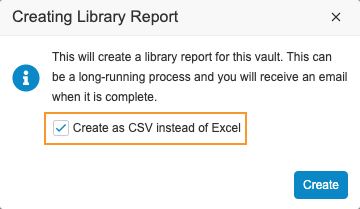
Create Library Report as CSV
Use Case
Excel™ has a million record limit. Having the report in a tab-delimited format allows for the processing of the Library Report in other tools, such as Microsoft Access™.
Description
With this release, librarians can now create the Library Report in tab-delimited format (with a .csv extension), instead of an Excel™ file. Vault automatically uses this format if there are more than 800,000 records in the Definition Histories object and will not give users the choice to export as an Excel™ file.
Enablement & Configuration
This feature is available automatically to users with permission to create a library report.
Option to Include Keys & Origin in SDS
Use Case
This feature provides users with an easy way to retrieve details about the origins of objects in their Study, as well as tie-ins to external systems using Veeva-managed keys. For example, the Origin Key (origin’s Cross Vault Unique ID) could be used to tie that definition to the metadata repository.
Description
Studio users can choose to include additional key values in their SDS to use for troubleshooting or tracking definitions across other applications, such as a metadata repository.
The available options include:
- Include Keys: This option adds columns for Cross Vault Unique ID and Private Key. The Cross Vault Unique ID provides the vault, study, and record IDs and is unique to the version of the object. The Private Key is used to define the object across versions and studies.
- Include Origin: This adds columns for origin keys. This set of keys tie the object to its point of origin, typically the library. These include the Origin Name (name of the study or library collection), Origin Type (either Study or Library), the Origin Definition Name (the current Name of the object in the origin study or library), and the Origin Key (the Cross Vault Unique ID tying the object to the Origin).
Enablement & Configuration
Contact Veeva Support to enable this feature. Studies created prior to the 21R1 release (initial release of the Library) may have missing origin records. Studies created after the 21R1 release have all origin records.
Show Codelist Labels in Email Notifications
Description
Send Email rules that contain references to codelist items now display the codelist label in addition to the backend coded value. Values are now displayed in the subject line and email body as “CODED VALUE(Label Value)”.
Enablement & Configuration
This change applies automatically.
Automatic Removal of Invalid Event Window Configuration for First Event
Use Case
This removes labor to fix a configuration that wasn’t available for the newly moved Event.
Description
This includes minor enhancements to the schedule design in Studio. When the user moves an existing Event as the first event in the schedule the system will now automatically remove the invalid window reference.
Enablement & Configuration
This feature is auto-on and available for all studies.
Unit Item Enhancements
Description
With this release, the error message for Unit Items has been updated for clarity and consistency with other number fields. Negative signs will no longer count as characters in a Unit Item. The default length and precision for unit items has been updated to 14 and 0.
When an item is linked with a form with display items that are units or global lab units, the units will display in Detail PDFs, Closeout PDFs, and Assessment PDFs.
Enablement & Configuration
These enhancements are available automatically.
Study Administration
Features in this section apply to EDC Tools, a study-level administration area for Vault EDC.
Principal Investigator No Longer Required for Site Creation
Description
The Site Principal Investigator field is no longer required when creating a new Site.
Enablement & Configuration
These enhancements are available automatically.
Include Freeze, Lock & Sign Dates in the Form Progress Listing for DM1 Studies
Use Case
This increases consistency with Data Model 2 studies.
Description
For studies on Data Model 1, the Form Progress Listing will now include values in the following columns:
- Freeze Date
- Lock Date
- Sign Date
These columns were previously only populated for Data Model 2 studies.
Enablement & Configuration
This change applies automatically in Studies using Data Model V1.
Job & Rule Execution Enhancements
Use Case
Users also have the ability to delineate between jobs that have been initiated but have not begun processing and are shown a completion percentage and timestamp for the last job fragment activity.
Description
With this release, we have removed the casebook limitation in the Run Rules job and doubled the amount of rules that can be run within one job. In addition, we have updated the EDC job retention policy to 30 days.
Enablement & Configuration
These changes apply automatically for all bulk EDC jobs.
New Column for All Items in the Form Progress Listing
Use Case
This feature allows users to determine how many items on the form have data, compared to the total number of items.
Description
A new column for All Items will now be available in the Form Progress Listing. This column will be included in the listing when “Include Item Counts” is selected in the job options dialog. The purpose of this column is to count the total number of items on the form, allowing users to compare this value with the number of items with data and the number of items with data change.
Enablement & Configuration
This feature is automatically enabled for Data Model 2 studies.
Notify Users when a Site is Locked from Review Plan Assignment
Description
With this release, Vault notifies users that a site is locked from EDC Tools > Review Plan Assignment.
Enablement & Configuration
These enhancements are available automatically.
Show Progress Percentage for In Progress Jobs
Use Case
Users also have the ability to delineate between jobs that have been initiated but have not begun processing and are shown a completion percentage and timestamp for the last job fragment activity.
Description
Users can see the progress completion percentage of In Progress jobs as the job runs.
Enablement & Configuration
These changes apply automatically for all bulk EDC jobs.
SDE Enhancements
Use Case
These enhancements support cross-functional features and add more information to SDE datasets.
Description
The following changes have been made to the 22R3 version of the SDE:
- The SYS_PD dataset will include Category, Subcategory, and Severity Labels along with Form Sequence, Form VOF ID, and Protocol Deviation VOF ID.
Enablement & Configuration
These enhancements are auto-on for the 22R3 version of the Study Data Extract job.
SDE: Include Study Design Metadata
Use Case
Users will be able to use study design information in tandem with clinical data for their downstream analysis.
Description
The 22R3 version of the SDE will have the option to “Include Study Design” in the New Job dialog. When checked, Vault will add a “definitions” folder to the zip package which contains CSVs with study design information.
Enablement & Configuration
This feature is automatically available for the 22R3 version of the Study Data Extract job.
SDE: Length Standardization
Use Case
Standardizing text column lengths across the SDE will prevent data value truncation.
Description
In the 22R3 version of the SDE, instead of defaulting SAS text column lengths, Vault will use Vault and Studio configuration data to determine column lengths.
For 22R3, codelist Decodes (Labels) will use 256 characters for length and 1024 bytes as the column length. In 22R2 and earlier versions, these will use 1500 characters for length and 1500 bytes as the column length.
Enablement & Configuration
This feature is automatically available for the 22R3 version of the Study Data Extract job.
Select Sites Across Multiple Pages when Running a Retrospective Amendment
Description
Users can select multiple Sites across pages when selecting Sites for a Retrospective Amendment.
Enablement & Configuration
These enhancements are available automatically.
Study Progress Listings in Reports
Use Case
This feature provides the ability to customize and filter Study Progress Listings.
Description
Study Progress Listings are now available in the Reports tab. To populate the reports with data, a user will need to schedule a listing job in EDC Tools and select the option to send the data to Vault Reports. Veeva recommends scheduling this listing as a daily job to ensure that the latest data is available in the reports.
As part of the job, Vault sends the listing data to the appropriate report. In the Reports tab, there are standard templates that match the output of the listing from EDC Tools. Users with access to Reports can create their own version of the report based on the standard template, which they can customize by adding filters or by adding or removing columns.
We added the following objects to support this feature:
- Event Progress Listing (
event_progress_listing__v) - Form Progress Listing (
form_progress_listing__v) - Query Detail Listing (
query_detail_listing__v) - Subject Progress Listing (
subject_progress_listing__v)
Enablement & Configuration
Contact Veeva Support to enable this feature in your vault.
Deployments
Features in this section are enhancements to deployment functionality in Vault CDMS.
Vault Diff Report & Multi-Role Security
Use Case
With the introduction of Multi-Role Security, the Vault Diff Report’s User Defined Roles section does not accurately reflect custom roles, instead displaying differences in the Security Profiles which is less applicable with the MRS model. This feature restores the ability of the Diff Report to provide accurate role and permission information regardless of the security model.
Description
Deployment Administrators can now run the Vault Diff Comparison Report and see details of roles and permission differences between Vaults, whether those Vaults are using Multi-Role Security or not.
Enablement & Configuration
This feature is auto-on. Customers using Multi-Role Security in all of their Vaults will immediately see their custom role and permission differences listed in the report. Customer’s not yet using Multi-Role will see the same Vault Diff Report as they currently do.
Labs
Features in this section are new features for the Labs module of Vault EDC.
Labs: Configurable Queries
Use Case
This feature provides more control and flexibility for Sponsors and CRO’s over system-generated Lab queries.
Description
With this release, users can enable or disable the following system-generated Lab queries:
- Lab Units Do not Match
- Lab Age Calculation
- Missing Lab Results
Enablement & Configuration
This feature is available automatically in Studies using Labs.
Labs: Global Lab Units & Codelists Display in UI Grids
Use Case
This feature provides consistency with other data entry grids.
Description
For studies using Global Labs, the Lab Units and Codelists will display in the following grids:
- Repeating Form Grid
- Form Linking Grid
- Item Linking Grid
Enablement & Configuration
This feature is available automatically in Studies using Labs.
Clinical DataBase (CDB)
The following are new features for the CDB application, the Vault CDMS solution for data cleaning and reporting.
Availability: Clinical DataBase (CDB) is only available to CDB license holders. Contact your Veeva Services representative for details.
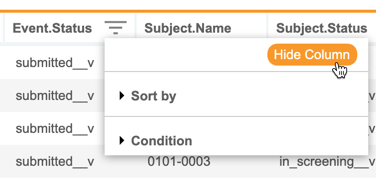
Hide Columns in Listings, Checks & Views
Use Case
This feature provides a better review experience for data managers with smaller displays.
Description
With this release, CDB users can now hide and show columns in all core and custom listings, checks, and views. The Sort & Filter menu now has a Hide Column option. When a column is hidden, CDB displays an orange, dotted line in place of the column. Once a user hides at least one column, CDB shows the Unhide Columns button, which includes a count of how many columns are hidden.
As part of this enhancement, users no longer need to click OK to apply a sort order from the Sort & Filter menu.
Enablement & Configuration
This feature is enabled automatically to all users.
Multiple Schedules per Export Definition
Use Case
CDB users can have greater flexibility when scheduling exports.
Description
CDB users can now have multiple scheduled exports per Export Definition. The Properties panel includes an Add Schedule button when there is an existing schedule.
Enablement & Configuration
This feature is enabled automatically to all users who can create and modify Export Definitions.
Reduce Header & Unit SAS Lengths in CDB Exports
Use Case
By reducing the SAS lengths of text column headers, users can save space when allocating memory in their downstream systems to ingest export data.
Description
CDB now reduces the SAS lengths of column headers in exports. Prior to this release, the length limit was defaulted to 6000 bytes. This change applies to column headers in core and system listings, as well column headers for all unit columns.
The following @HDR, @Form, @ItemGroup, and Item property columns were reduced from 6000 to 512 bytes:
- @HDR.Study.Name
- @HDR.Site.Name
- @HDR.Site.Number
- @HDR.Site.Country
- @HDR.Site.PI
- @HDR.Subject.Name
- @HDR.Subject.Status
- @HDR.EventGroup.Name
- @HDR.Event.Name
- @HDR.Event.Date
- @HDR.Event.Status
- @Form.Name
- @Form.Status
- @Form.ExternalID
- @ItemGroup.Name
- Name (on the Item)
The following ILB reason property columns were reduced from 6000 to 1020 bytes:
- @Form.ILBReason
- ILBReason (on the Item)
The following unit columns were reduced from 6000 to 512 bytes:
- Item_UOM
- Item_UOM_TRANSLATED
Enablement & Configuration
This change applies automatically to all exports.
CDB Support for Item to Form Linking
Use Case
Users can create custom listings that include Item to Form Links using CQL.
Description
CDB now supports Item to Form Linking by using the newly defined @FormLink notation.
Enablement & Configuration
This feature is enabled automatically.
Role Management & Security
Features in this section are enhancements to the System Tools > Role Management and System Tools > Users areas, as well as changes to standard Study Roles, security, and access control in Vault CDMS.
User Import Enhancements
Description
With this release, we have introduced a number of enhancements to manage Users and Roles, including the following:
- Users will be warned when editing an existing user’s details when importing
- Users will be warned when overlapping site and country access is specified when importing users
Enablement & Configuration
These enhancements are available automatically.
User Training Report Includes Users with All Sites Access
Description
The User Training Report now includes Users with All Sites access.
Enablement & Configuration
These enhancements are available automatically.
Tooltip for Add as PI Field
Description
In System Tools > Users, the Add as PI field now includes an explanatory tooltip.
Enablement & Configuration
These enhancements are available automatically.
Integrations
Features in this section are new integrations with Vault CDMS or enhancements to existing integrations.
E2B Link: Configuration Deployment
Use Case
Now that the DEV environment is the only environment that allows configurations, organizations will enjoy a controlled and uniform configuration across their study environments. This allows for transparency in what is available in the E2B Link Safety Configuration during the UAT and production phases of the Study.
Description
E2B Link’s Safety Configurations now must be configured in a development (DEV) study environment and then deployed to downstream UAT and production environments.
During deployment, the provided validation file now includes validation checks of the Safety Configuration. If there is a situation in the configuration that would prevent the E2B XML from being generated or from including expected relevant case data, then that will show up in the validation file as either an error or a warning.
Enablement & Configuration
This feature is available automatically.
Feature Enablement Summary
| Feature Name | Configuration | Dependencies | Day 1 Impact to Primary Users | Users with Day 1 Visibility |
|---|---|---|---|---|
| Data Entry | ||||
| Default Settings for Data Entry |
|
|
||
| Data Review | ||||
| Ability to Specify Other Reason for Additive Review | Additive Review |
|
|
|
| Protocol Deviations Enhancements | Protocol Deviations |
|
|
|
| Protocol Deviations: Export as an Excel™ File | Protocol Deviations |
|
|
|
| Clinical Coding | ||||
| Coder UI Enhancements |
|
|
||
| Coders are Allowed to Code a Form with a "Needs Synonym List" Status |
|
|
||
| New Upversioning Status in Coder |
|
|
||
| Study Administration | ||||
| Principal Investigator No Longer Required for Site Creation |
|
|||
| Include Freeze, Lock & Sign Dates in the Form Progress Listing for DM1 Studies | Data Model V1 |
|
||
| Job & Rule Execution Enhancements |
|
|||
| New Column for All Items in the Form Progress Listing | Data Model V2 |
|
||
| Notify Users when a Site is Locked from Review Plan Assignment |
|
|||
| Show Progress Percentage for In Progress Jobs |
|
|||
| SDE Enhancements |
|
|||
| SDE: Include Study Design Metadata |
|
|||
| SDE: Length Standardization |
|
|||
| Select Sites Across Multiple Pages when Running a Retrospective Amendment |
|
|||
| Study Progress Listings in Reports | Vault Admin | |||
| Study Design & Configuration | ||||
| Create Library Report as CSV |
|
|||
| Show Codelist Labels in Email Notifications |
|
|||
| Automatic Removal of Invalid Event Window Configuration for First Event |
|
|||
| Unit Item Enhancements |
|
|||
| Labs | ||||
| Labs: Configurable Queries | Labs |
|
||
| Labs: Global Lab Units & Codelists Display in UI Grids | Labs |
|
||
| Deployments | ||||
| Vault Diff Report & Multi-Role Security | Multi-Role Security |
|
||
| Integrations | ||||
| E2B Link: Configuration Deployment |
|
|||
| Role Management & Security | ||||
| User Import Enhancements |
|
|||
| User Training Report Includes Users with All Sites Access |
|
|||
| Tooltip for Add as PI Field |
|
|||
| Vault CDB | ||||
| Hide Columns in Listings, Checks & Views |
|
|||
| Multiple Schedules per Export Definition |
|
|||
| Reduce Header & Unit SAS Lengths in CDB Exports |
|
|||
| CDB Support for Item to Form Linking |
|
|||
Enablement Legend
- Configuration: This field lists the location(s) where configuration for this feature occurs, for example, "Studio" or "EDC Tools". "Support" indicates that this feature must be enabled by Veeva Support, and "Vault Admin" indicates that configuration must be performed by a Vault Owner in the vault's Admin area.
- Dependencies: This field lists any dependencies required to use this feature, for example, Labs or Expression Engine V2. The other columns assume that the dependencies are enabled/in use.
- Day 1 Impact to Primary Users: This feature is visible and available to one or more primary user teams (Site Users, Clinical Team, and Coders) on day 1. Otherwise, this feature is either only visible to study designers or administrator users, it requires configuration before it is visible to primary users.
- Users with Day 1 Visibility: This feature is visible to these users on day 1 if no configuration occurs.