New Features in 22R3.4
Rules Archive, SAE Not Submitted Alerts, and more...
Release Date: February 10, 2023
We are pleased to bring you the following new features in this week's release. See a summary of feature enablement for this release below. Information on developer features (REST API) is in the Developer Portal.
Data Entry
Features in this section are changes to the Data Entry tab, a working area for investigators and clinical research coordinators to enter study execution data.
Study Closeout: Non-restricted Version
Use Case
Users no longer need to create custom roles to download and accept non-restricted Closeout PDFs.
Description
With this feature, Vault will check if a site contains any restricted forms when generating Closeout PDFs. The Closeout PDF won’t be marked as restricted if no restricted data is detected. Site users won’t be required to have the restricted data permission in order to download and accept the files.
Enablement & Configuration
This feature is available automatically.
Clinical Coding
The following are new features for Coder, the clinical coding area for Vault Coder.
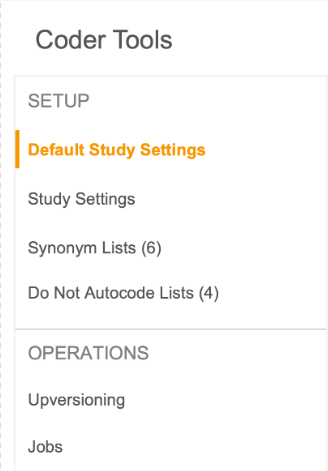
Coder Tools Navigation Refresh
Use Case
This new UI experience is scalable as we add more features to Coder Tools.
Description
With this feature, the main navigation in Coder Tools will be moved to the left panel.
Enablement & Configuration
Auto-on.
UI for Orphaned or Deleted Coder Items
Use Case
Study Designers will have options to fix the Coding Configuration instead of requiring assistance from Veeva Support.
Description
With this release, Study Designers can still see coding configurations for Form and Item Definitions from an orphaned Verbatim Item Definition or an orphaned or deleted related coding Item Definition. Study Designers can then select a new Item Definition or make the mapping not required (in the case of related Coding Item Definitions only). Users can also delete the Coding Configuration on the Form Definition entirely.
Enablement & Configuration
Auto-on.
Study Design & Configuration
Features in this area apply to Studio, the study design and configuration area for Vault EDC.
Remove Subject Status Date on Status Rollback
Use Case
This change improves support for CTMS-integrated Studies.
Description
As a subject progresses through a Study, Vault updates its Subject Status. When a subject’s status changes, Vault records the date that a subject entered that status. With this release, when data changes for a subject, if a Set Subject Status Rule that previously evaluated as true is now false, Vault clears the associated status change date.
This feature adds a new setting to Studio > Settings, Enable Subject Status Date Rollback.
Enablement & Configuration
For existing Studies (created before 23R1), a study designer must enable this feature from Studio > Settings. For new Studies created after 23R1, this feature is automatically enabled.
Learn More
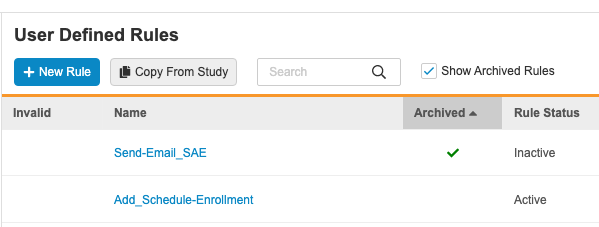
Rules Archive
Use Case
This simplifies the deployment of rules. Users are now also able to restore deleted rules and view deleted rules for reference purposes.
Description
When deleting rules, Vault now marks those deleted rules as Archived. After a user executes the Delete action against a Rule, Vault applies the Archived status, marks the rule as Inactive, and hides the rule from the User Defined Rules listing. Studio users can select the Show Archived Rules checkbox to show archived rules in the list. Archived rules have a green checkmark () in the Archived column.
Users can then view and restore these archived rules. Archived rules behave in the same way as Inactive rules. Vault excludes these rules from validations and documentation, deploys them (in the Archived status), and prevents users from marking them as Active. That prevents these rules from executing.
Enablement & Configuration
Contact Veeva Support to enable this feature in your vault.
Learn More
SDS Enhancements
Use Case
This feature provides better usability of the SDS.
Description
This release includes the following SDS enhancements:
- Added columns for missing properties and system edit checks to the Schedule Tree tab and updated column headers and order of columns for consistency
- Added missing columns to the Form Definitions tab, relabeled column headers to match UI, and removed obsolete/retired columns
- Added Last Modified columns to Form Definitions and Schedule Tree
- The “Include Rules” option is now checked by default when generating an SDS from Studio
- Updated formatting of the Review Plan tab including newly-formatted rows for different object types to match the Form Definition tab and carried down column values for filtering
- Removed obsolete columns and added a Form Label column in the Rules tab
- Added Form Name and column headers to Schedule Grid tab
- Updated the Summary tab for clarity
- Added and updated columns on the Codelists and Unit Codelists tabs
- Added Object Name columns to the Date Comparisons tab
Enablement & Configuration
These changes apply automatically.
Studio Usability Improvements
Use Case
These changes improve the usability of Studio to speed study build delivery.
Description
This release includes several usability improvements for Studio:
- Schedule is now the default landing page when opening Studio.
- The Comparison Report (diff report) no longer reflects changes to the rule action order that are controlled by the system.
- We made some minor formatting, processing, and logging changes to export and import for study languages.
- We relabeled the Future Date property as No Future Date to clarify the purpose of this property.
- The default value for Repeat Maximum is now “50” instead of “9999”.
- Studio users can edit library settings and some study settings after a Study is deployed.
- If no Form Links are included in the study’s schedule, Studio doesn’t run validations against Form Links.
- We added validation errors for unsupported uses of cross-form derivations.
- Cross-form derivation rules that cause a circular reference between items will trigger a warning instead of a casebook validation error when the circular reference exists only within a single form.
Enablement & Configuration
These changes apply automatically.
Study Administration
Features in this section apply to EDC Tools, a study-level administration area for Vault EDC.
Disable Manual Casebook Creation
Use Case
Customer’s with IRT (Interactive Response Technology)-enabled studies need a way to block casebook creation through the EDC UI. For these studies, new casebooks should only be created from external integration. This feature removes the need to create custom roles without the Add Casebook permission.
Description
EDC Tools now provides a setting for IRT-enabled studies that disables the + New Casebook button in the Data Entry tab. When the Disable Manual Casebook Creation checkbox is selected for a study, new casebooks can only be created via the external IRT integration. Users must have the Edit Study Settings permission to modify this setting.
Enablement & Configuration
Contact Veeva Support to enable this feature in your vault. Once enabled at the vault level, this feature must be enabled in a Study via the Disable Manual Casebook Creation checkbox in Tools > EDC Tools > Settings.
Removed Actions Menu from LMS External Connections
Use Case
In the past, there has been a Actions menu icon (gear) denoting an Actions menu next to the LMS connection record that did not function. This icon has been eliminated to remove confusion.
Description
With this release, we removed the extraneous Actions menu icon from System Tools > External Connections > LMS External Connections.
Enablement & Configuration
This feature is available by default.
Review Plan Assignment UI Enhancements
Use Case
These enhancements provide improved navigation and usability of the Review Plan Assignment section, and allow for more granular permission access to the Review Plan Assignment criteria and manual access pages.
Description
With this release we have introduced two new tabs to EDC Tools, Review Plan Assignment Criteria and Review Plan Manual Assignment, that take the place of the single Review Plan Assignment tab and subtabs. In addition, users can now search for site number in addition to site name in a Review Plan Assignment Sites field.
Enablement & Configuration
These changes apply automatically to Studies using V2 or V3 of Review Plan Assignment.
SDE Enhancements
Use Case
These enhancements add more detail to the extract to help users with post-processing analysis of their clinical and operational data.
Description
The following enhancements have been made to the 23R1 version of the SDE:
- Users will have the option to select a boolean formatting type which will determine how booleans are displayed in both clinical and system datasets. This formatting doesn’t apply to the definitions files
- If there are duplicate column headers, both the CSV and SAS files will follow a deduplication pattern of appending _2, _3, etc. to each additional repeating column. When running the “SAS with CSV and XPT”, Export File Type option, users will see an additional column for “SAS Column” in the Study Definitions file so that they can compare the column headers between CSV and SAS for any differences (such as SAS column header truncation for column headers greater than 32 characters in length)
- The rows in the casebook_schedule.csv and casebook_definition_summary.csv files in the definitions folder will be ordered according to the studio study Schedule Design within each casebook version
- The SYS_Q dataset will have a new column for the LASTCLOSEDDT (Query Last Close Date)
Enablement & Configuration
Auto-on with the 23R1 version of SDE
SDE: New Lab Form Format
Use Case
This format makes Lab forms more readable for reviewing clinical data.
Description
In the 23R1 version of the SDE, clinical forms configured with Local Labs will be formatted to decrease the number of columns outputted so that the dynamically-generated Labs columns will be shared across all Lab Analytes on the form instead of having multiple columns per analyte.
Enablement & Configuration
Auto-on with the 23R1 version of the SDE
Study Progress Listing Enhancements
Use Case
With this feature, Vault cleans up additional jobs after the study priority expires.
Description
Users can schedule up to one daily listing job to send to reports, unless the study is set as a priority study. Users can schedule two additional daily jobs for priority studies. When the study’s priority expires, Vault will also automatically expire the additional jobs.
Enablement & Configuration
These changes apply automatically in Studies using the Study Progress Listings in Reports feature.
Deployments
Features in this section are enhancements to deployment functionality in Vault CDMS.
Deploy from Development to Validation Environments
Use Case
Previously, the only deployment option to Validation was from UAT. With this feature, deployment administrators can save time by going directly from DEV to UAT.
Description
With this release we have provided the option to deploy from Development environments directly to Validation.
Enablement & Configuration
This feature is automatically enabled.
Prevent Post-Deployment Removal of Roles from Deployment List
Use Case
Previously, in vaults using the Multi-Role Security model, deployment administrators could inadvertently remove a custom role from a target vault if a previously deployed role was removed from the whitelist before a subsequent deployment. This feature removes this possibility by ensuring that a custom role cannot be removed from the whitelist if it has already been deployed.
Description
This feature prevents the unintended removal of custom roles during a vault deployment by disabling the ability to remove a custom role from the deployment list if it has already been deployed.
Enablement & Configuration
This feature is enabled by default for all vaults using the Multi-Role Security model.
Labs
Features in this section are new features for the Labs module of Vault EDC.
Labs: Support for Multiple Languages
Use Case
Local labs can support multiple languages.
Description
Users can upload translated values for codelists, units, and analytes in Local Labs.
Enablement & Configuration
This feature is available automatically.
Clinical DataBase (CDB)
The following are new features for the CDB application, the Vault CDMS solution for data cleaning and reporting.
Availability: Clinical DataBase (CDB) is only available to CDB license holders. Contact your Veeva Services representative for details.
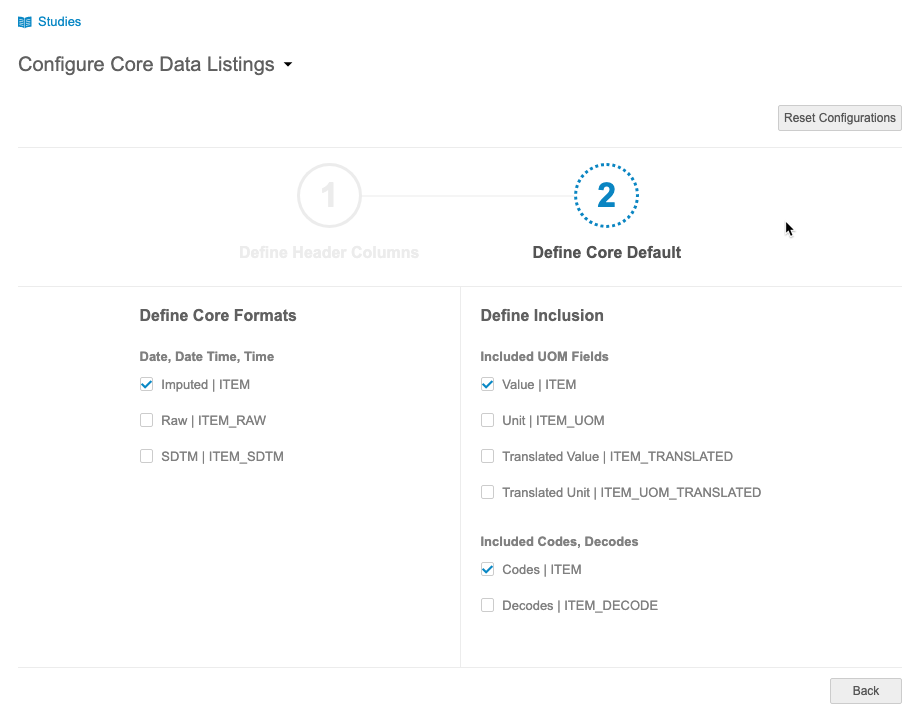
Configure Core Data Listings
Use Case
Core data listings are out-of-the-box listings that correspond to each Form represented in the study. Data managers may prefer a specific format and specific set of study attributes be included in all of Core Listings. With the ability to configure these sets at a vault level, clinical programmers will save a substantial amount of time by not having to recreate each core listing individually.
Description
Administrators can now configure Core Listings. This configuration includes the ability to select which study attributes to include and which formats to use, such as always using raw format for datetimes or always including Translated Value and Translated UOM. Configuration is subject to a review and approval process.
Configurations apply to all Core Listings within a vault.
This feature adds a new Configure Core Data Listings page to CDB.
Enablement & Configuration
The ability to configure core data listings is automatically enabled. For existing listings, the default configuration remains the same. An administrator must review and approve configuration changes before CDB applies the changes.
Listing Search & Favorites
Use Case
Navigation is an essential part of the user experience and enables data managers to access data as quickly and intuitively as possible.
Description
With this release, CDB users can mark listings, checks, and views as Favorites. They can manage their favorites from the Favorites panel, which is accessible via the Favorites () button in the top navigation menu.
|

|
Users can now also search for listings from any page within the application. Search has moved into the top navigation bar. Prior to this release, users could only search from the Listings page.
Enablement & Configuration
This feature is automatically enabled.
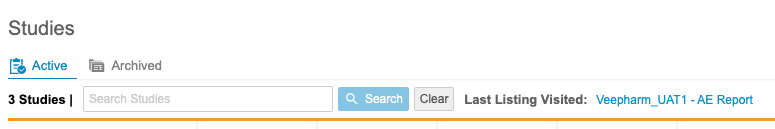
Study Search & Last Listing Visited
Use Case
Navigation is an essential part of the user experience and enables data managers to access data as quickly and intuitively as possible.
Description
Users can now search for specific Studies from the Studies page in Workbench. Workbench also displays a link to the Last Listing Visited on this page, which opens the last listing that the current user visited.
Enablement & Configuration
This feature is automatically enabled.
Open Listing in a New Tab
Use Case
Users may need to access multiple listings at one time. With this feature, it’s easier for users to compare different sources of data quickly and efficiently.
Description
With this release, CDB allows users to access multiple listings simultaneously across different browser tabs. Users can open a listing in a new tab.
Enablement & Configuration
This feature is automatically enabled.
Export Audit Logs Track Blinding Changes
Description
CDB now tracks the following changes to package blinding in the audit log for Export Definitions:
- Export definition marked as Blinded
- Export definition marked as Unblinded
Enablement & Configuration
This feature is available automatically. Any changes to package blinding are captured by the audit log following this release.
Role Management & Security
Features in this section are enhancements to the System Tools > Role Management and System Tools > Users areas, as well as changes to standard Study Roles, security, and access control in Vault CDMS.
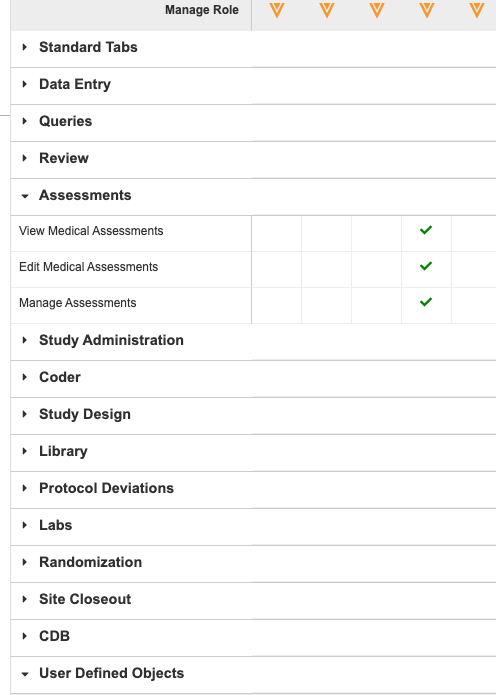
Permission Grouping in Role Management
Use Case
The Role Management grid contains many permissions and has been hard to navigate and find relevant permissions without an inordinate amount of vertical scrolling. This UI enhancement will greatly improve the UX of the Role Management grid, reducing frustration and time spent navigating.
Description
With this release, we have introduced grouping to the Role Management grid in System Tools. Users can expand and collapse these group sections to more easily navigate and find the correct permissions.
We replaced the Permissions section with multiple, categorical sections:
- Data Entry
- Queries
- Review
- Assessments
- Study Administration
- Coder
- Study Design
- Library
- Protocol Deviations
- Labs
- Randomization
- Site Closeout
- CDB
Enablement & Configuration
These changes apply automatically.
User Activity Report Changes
Description
We removed information related to user access (the Current Study Role, Study, Item, Old Value, and New Value columns) from the User Activity Report in preparation for a feature in a later release.
We also added the following tracked events for this report:
- User Unlocked: The user successfully reset their password after being locked out.
- Password Change Unsuccessful: The user attempted to change their password but was unsuccessful.
- Logout Successful: The user logged out of Vault successfully.
- User Inactivated: The user attempted to log in to an inactive account.
Enablement & Configuration
These changes apply automatically.
Study Role Enhancements
Use Case
These enhancements provide permissions to new sections of the application and ensure that roles are able to access and interact with the proper functionality.
Description
With this release, we made the following changes to permissions and standard Study Roles:
- Added the CDB Tools permission and assigned it to the CDMS Data Manager and CDMS Lead Data Manager roles
- Added the Configure CDB permission and assigned it to the CDMS Super User role
- Added the Manage Review Plan Assignment Criteria and Manage Review Plan Manual Assignment permissions
- Assigned the Manage Jobs permission to the CDMS Clinical Research Associate and CMDS Data Manager roles
- Assigned the View Users permission to the CDMS API Read Only and CDMS API Read Write roles
- Assigned the Edit Users permission to the CDMS API Read Write role
Enablement & Configuration
These changes apply automatically.
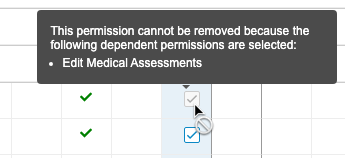
Display Permission Dependencies in Role Management UI
Use Case
Prior to this release, when creating or editing a custom role, users couldn’t easily determine why some permissions were automatically selected, or could not be cleared. This feature provides information on why a certain permission or permissions were added and can’t be removed.
Description
This feature provides a tooltip in the editable Role Management grid when permissions are dependent on an already selected permission.
Enablement & Configuration
This feature is available by default.
Updates to Inactive Users
Use Case
Automatically removing a user’s study access when they have been inactivated will cut down on extraneous clicks, and will ensure that a User Admin does not inadvertently provide study access upon subsequent activation of a user. In addition, previously a user admin would receive a vague error message when attempting to edit an inactivated user’s details. This feature now removes that ambiguity and simply disables the ability to edit the profile of an inactive user.
Description
With this release, the system will automatically remove a user’s study access when they have been inactivated, and will disable the ability to edit an inactivated user’s details.
Enablement & Configuration
These changes apply automatically.
User Import Enhancements
Use Case
The system has always allowed changes to a user’s activation date in individual user management as long as the activation date is not in the past. This feature extends the same behavior to the bulk user import from file feature.
Description
This feature allows user administrators to make changes to a user’s Activation Date creating users via import from file. In addition, if an Activation Date is not specified, Vault uses the current date and indicates that in a warning during import preview.
Enablement & Configuration
These changes apply automatically.
Integrations
Features in this section are new integrations with Vault CDMS or enhancements to existing integrations.
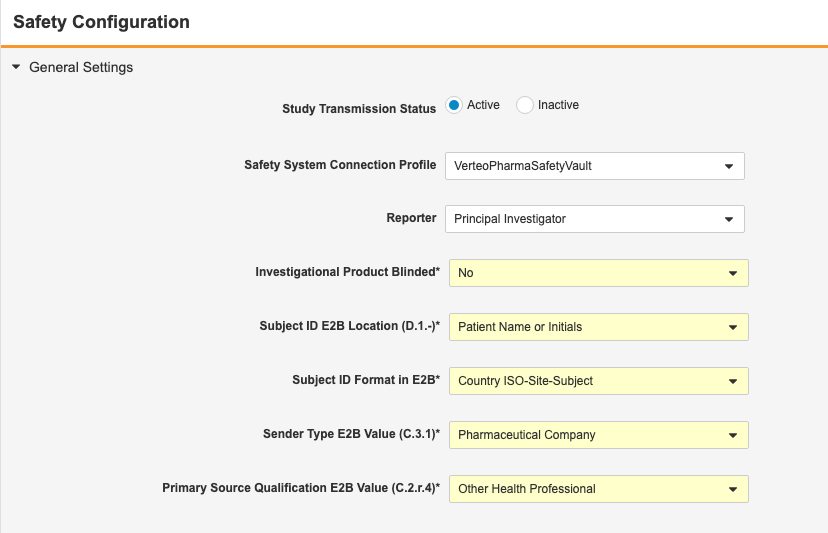
E2BLink: Configure Static Values in E2B XML
Use Case
Safety administrators have further control over what information is included in the safety case.
Description
Prior to this release, E2BLink transferred several static values to the safety system. With this release, safety administrators can configure which values are sent, instead of the CDMS default values for these fields.
The following values are now configurable:
| Field | E2B ID | Default Value | Description |
|---|---|---|---|
| Subject ID E2B Location | D.1 | Patient Name or Initials | Location of the EDC Subject ID in the E2B XML files |
| Subject ID Format in E2B | N/A | Country ISO-Site-Subject | Format of the EDC Subject ID in the E2B XML files |
| Sender Type E2B Value | C.3.1 | Pharmaceutical Company | Qualification type of the assessment of the drug, as it relates to adverse events for the case |
| Primary Source Qualification E2B Value | C.2.r.4 | Other Health Professional | Specialty classification of study drugs, when a site user has indicated that the concomitant medication (in the case data) is classified as Suspect |
| Study Drug Classify when ConMed Suspect | G.k.1 | Always Interacting | For cases when a user is classifying a Concomitant Medication via an Item to Form link, and user chooses SUSPECT, then this classification is to be instead used for Study Drug classifications of the Safety Case. |
Enablement & Configuration
Contact Veeva Support to enable this feature.
Learn More
EDC Migrator
Features in this section are new features for Veeva EDC Migrator.
Create Repaired Forms as Facades
Use Case
When Forms are placed “In Progress” there is the implication to Users that they must submit the Form. With this enhancement, Users can leave the Forms as Facades and not have to submit them. Furthermore, when a Form is first created in EDC, it’s created as a Facade, so this behavior would mimic existing EDC functionality.
Description
This update to the Repair process ensures that all Forms created by repair are created as Facades (blank forms). Prior to this release, these forms were in the In Progress status.
Enablement & Configuration
This is automatically enabled and can’t be disabled.
Migration: UI Redesign - New Rules Steps
Use Case
With these enhancements, users are no longer required to execute the rules one batch-at-a-time. This feature automates the Rules process, saving users from having to spend significant time executing and monitoring the lengthy process.
Description
These enhancements to the Load workflow automate the rules steps of the migration process.
Enablement & Configuration
This feature is automatically enabled. Migration users have the option to skip these optional steps.
Notify Users after Step Completion
Use Case
Users need to be notified after any of the steps are complete so that they know when and if the next step can begin.
Description
This feature introduces two new Notifications that are sent to Users after a Step has completed. One notification utilizes the system’s Notification Service, while the other one involves informing the user by email.
Enablement & Configuration
This is automatically enabled and can’t be disabled.
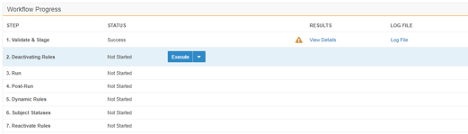
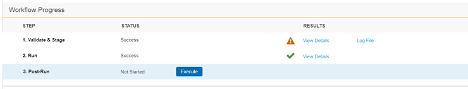
Migration: UI Redesign - Validate & Stage, Run, Post-Run
Use Case
This design makes the Load workflow more intuitive and makes the UI more user-friendly. Users can quickly review load progress with fewer clicks in the new Workflow Progress table.
Description
This release introduces a new design to make the workflow more intuitive and the UI more user-friendly. This feature focuses on the design of existing load workflow steps: Validate & Stage, Run, and Post-Run. Part of this design is the Workflow Progress table. This table does the following:
- Walks users through each Step of the Load workflow
- Provides controls to execute or skip each Step
- Provides a consistent user experience when viewing the results of completed Steps
- Provides quicker access and greater detail for any errors and warnings that may occur during a Step
This feature removes the extraneous Run step from the Load workflow, simplifying the Migration workflow.
Enablement & Configuration
This feature is automatically enabled.
Feature Enablement Summary
| Feature Name | Configuration | Dependencies | Day 1 Impact to Primary Users | Users with Day 1 Visibility |
|---|---|---|---|---|
| Data Entry | ||||
| Study Closeout: Non-restricted Version |
|
|
||
| Clinical Coding | ||||
| Coder Tools Navigation Refresh |
|
|||
| UI for Orphaned or Deleted Coder Items |
|
|
||
| Study Administration | ||||
| Removed Actions Menu from LMS External Connections |
|
|||
| Review Plan Assignment UI Enhancements | Review Plan Assignment V2 or V3 |
|
||
| SDE Enhancements | 23R1 SDE version |
|
||
| SDE: New Lab Form Format | 23R1 SDE version |
|
||
| Study Progress Listing Enhancements |
|
|||
| Study Design & Configuration | ||||
| Remove Subject Status Date on Status Rollback |
|
|||
| SDS Enhancements |
|
|||
| Studio Usability Improvements |
|
|||
| Labs | ||||
| Labs: Support for Multiple Languages | Labs |
|
||
| Deployments | ||||
| Deploy from Development to Validation Environments |
|
|||
| Prevent Post-Deployment Removal of Roles from Deployment List | Multi-Role Security |
|
||
| Role Management & Security | ||||
| Permission Grouping in Role Management |
|
|||
| User Activity Report Changes |
|
|||
| Study Role Enhancements |
|
|||
| Display Permission Dependencies in Role Management UI |
|
|||
| Updates to Inactive Users |
|
|||
| User Import Enhancements |
|
|||
| Vault CDB | ||||
| Configure Core Data Listings |
|
|||
| Listing Search & Favorites |
|
|||
| Study Search & Last Listing Visited |
|
|||
| Open Listing in a New Tab |
|
|||
| Export Audit Logs Track Blinding Changes |
|
|||
| Integrations | ||||
| EDC Migrator | ||||
| Limited Availability: In the current release, EDC Migrator is only available to early adopter customers. Contact your Veeva Services representative for details. | ||||
| Create Repaired Forms as Facades |
|
|||
| Migration: UI Redesign - New Rules Steps |
|
|||
| Notify Users after Step Completion |
|
|||
| Migration: UI Redesign - Validate & Stage, Run, Post-Run |
|
|||
Enablement Legend
- Configuration: This field lists the location(s) where configuration for this feature occurs, for example, "Studio" or "EDC Tools". "Support" indicates that this feature must be enabled by Veeva Support, and "Vault Admin" indicates that configuration must be performed by a Vault Owner in the vault's Admin area.
- Dependencies: This field lists any dependencies required to use this feature, for example, Labs or Expression Engine V2. The other columns assume that the dependencies are enabled/in use.
- Day 1 Impact to Primary Users: This feature is visible and available to one or more primary user teams (Site Users, Clinical Team, and Coders) on day 1. Otherwise, this feature is either only visible to study designers or administrator users, it requires configuration before it is visible to primary users.
- Users with Day 1 Visibility: This feature is visible to these users on day 1 if no configuration occurs.