New Features in 23R1.4
Snapshots: Bulk Lock & Freeze, Open EDC, and more...
Release Date: June 30, 2023
We are pleased to bring you the following new features in this week's release. See a summary of feature enablement for this release below. Information on developer features (REST API) is in the Developer Portal.
Clinical Coding
The following are new features for Coder, the clinical coding area for Vault Coder.
Display Third-Party Coding Suggestions
Use Case
With this feature, Coders can select from a list of curated suggestions rather than trying to locate the correct code directly from the dictionary. Coders can also delete suggestions on a form so that the third-party can generate and post new suggestions for the entire form.
Description
This feature displays third-party suggestions in the Coder UI, which will reduce the time users spend on locating the correct coding.
Enablement & Configuration
Contact Veeva Support to enable this feature.
Learn More
Study Administration
Features in this section apply to EDC Tools, a study-level administration area for Vault EDC.
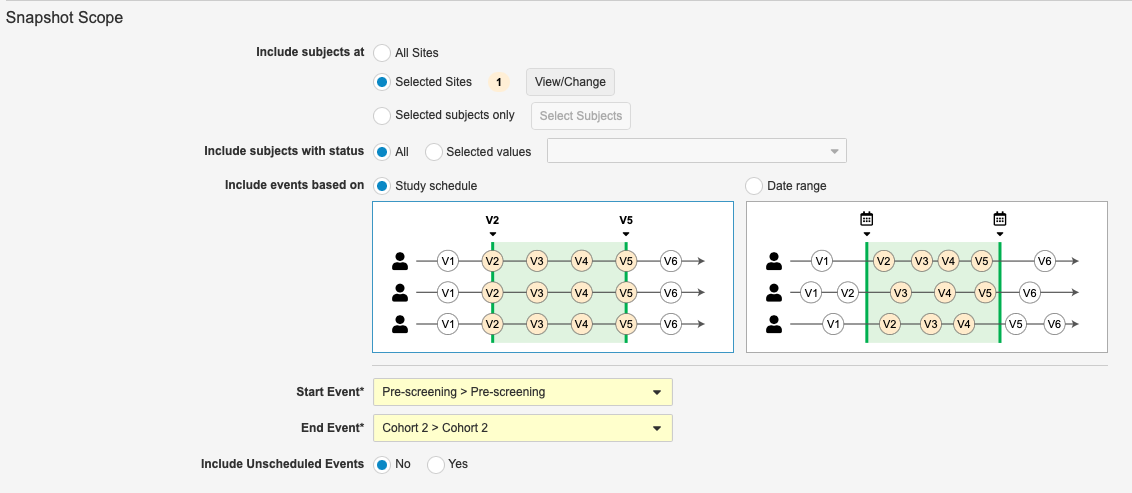
Snapshots: Bulk Lock & Freeze
Use Case
Some data managers lock data for interim milestones, so the enhanced “bulk action” capabilities using the new snapshots feature can provide a more efficient process.
Description
With this release, we’ve added a new tab, Snapshots, to the Review tab where Data Managers can create snapshots of clinical data for analysis during study milestones. Users can use specific filtering to define milestone criteria for the selected subset of data before performing lock/unlock and freeze/unfreeze actions in bulk. This feature also includes a Bulk Action Preview, which allows the user to select which sites and casebooks to preview. The Bulk Action Preview shows both data that is ready for bulk action (the data that meets the milestone criteria), and data that is not ready (data that is within the milestone but doesn’t meet all of the milestone criteria). The resulting bulk action job sends an email notification and provides a report detailing the subjects and events that were bulk actioned.
Enablement & Configuration
This feature is automatically available in Studies using Data Model V2.
Learn More
CDMS Billing Status Support
Use Case
This feature provides clients the ability to independently manage billing statuses for studies on hold, locked, or archived.
Description
This feature supports the tracking of a study’s licensing and billing status. The billing status of a study is set to Deployed automatically upon deployment to production. Through EDC Tools, an organization’s enabled users can modify the study status to On Hold, Locked, or Archived.
As part of this feature, we renamed the Manage Study Lock permission to Manage Study Milestones.
Enablement & Configuration
This feature is available immediately. Existing studies that do not yet have a Production Environment created will need to have the licensing status updated by Veeva. Updating Billing Status will be available for any role with the Manage Study Milestones permission. This feature only applies to production (PROD) study environments, and so it is only available in production vaults.
Learn More
Clinical DataBase (CDB)
The following are new features for the CDB application, the Vault CDMS solution for data cleaning and reporting.
Availability: Clinical DataBase (CDB) is only available to CDB license holders. Contact your Veeva Services representative for details.
Conditional Unblinding
Use Case
This feature allows selected data to be unblinded based on data within the import file from third party sources. For example, lab data may contain values from multiple tests. If some but not all of the test results need to be blinded, this feature allows users with the new Manage Unblinding Rules permission to configure a rule to unblind test results for those tests that do not need to be blinded.
Description
Conditional unblinding allows items from third-party data sources that are marked as blinded in the manifest to be unblinded based upon data within the same data set. Only one rule per data set is allowed, but the rule can unblind multiple items, and can have multiple conditions to determine if those items should be unblinded.
This feature introduces the Manage Unblinding Rules permission, which is assigned to the CDMS Super User and CDMS Lead Data Manager study roles by default.
Enablement & Configuration
This feature requires the Mask Blinded Data feature to be enabled at the Vault level. At the study level, rules can be configured from the new Sources page under Import. Rules are not deployable and must be configured for each study instance separately.
Learn More
Mask Blinded Data
Use Case
This feature displays the column to end users, while keeping the value of the data masked. This is required to enable Conditional Unblinding.
Description
CDB now provides the option at the Vault level to add a mask to blinded data. You can select to mask data in the UI, in exports, or both.
Enablement & Configuration
This feature must be enabled from CDB > Configurations. Enabling this feature does so for all Studies in the vault.
Learn More
OpenEDC: Support for Non-Veeva EDC Studies
Use Case
Customers who have studies outside of Clinical Data will be able to use a single system for data cleaning from all of their data sources.
Description
Open EDC allows customers using Legacy EDC systems, with studies outside of Clinical Data, the ability to clean their study data using CDB, by importing data from their EDC system into CDB. This feature utilizes the new External study type in Studio when creating a study instance.
This feature introduces the new Manage Sources permission, which is assigned to the CDMS Super User and CDMS Lead Data Manager study roles by default.
Enablement & Configuration
Contact Veeva Support to enable this feature.
Learn More
Integrations
Features in this section are new integrations with Vault CDMS or enhancements to existing integrations.
Safety Integration Follow Up Processing Changes
Use Case
Evaluation of medicinal product safety depends on reliable and up-to-date Safety Case data. Follow-up reporting must be assured for any change to any data of existing cases. A full re-run for follow-up reports allows for the consolidation of all Safety Cases on a regular basis.
With the release of the Duration Inclusion Criteria for Related Forms feature in 23R1 (April 2023), changes to a live study’s design could mean that previously sent Safety Cases now require a follow-up, based on the adjusted rules. With this feature, the scheduled full scan catches those case changes, or a user can run an ad hoc scans immediately after deployment of such changes.
Description
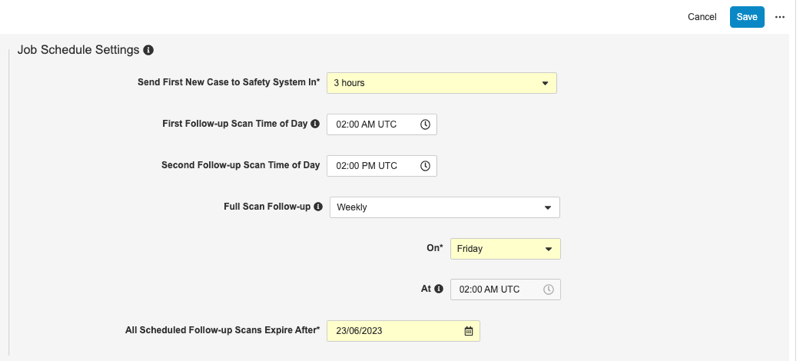
With this release, we made follow-up processing more robust, to assure the sending of a follow-up report with any data changes for existing Safety Cases. Vault now uses a recurring job, instead of an integration task on a timer, to do follow-ups. Users can configure this enhanced follow-up processing job to run daily or twice a day. They can also schedule a weekly or monthly scan of all cases, or run on an ad hoc basis, to resolve any potential issues with incremental follow-up reports.
Users with the Manage Safety Integrations permission can make schedule changes to this job from Tools > Safety Integrations > Study Settings in the Job Schedule Settings section.
Enablement & Configuration
This feature is automatically enabled. Veeva will contact customers to discuss this change in more detail. Each existing production Study that has the safety integration enabled will be configured to run the job once daily, at 2:00 AM UTC, and run a full scan monthly, on the 15th of the month, unless otherwise specified. Development, training, validation, and test (UAT) environments will not have any jobs automatically scheduled. In these environment types, this job is typically run on an ad hoc basis. Organizations can continue to review these job configurations and other safety settings as their study progresses from Tools > Safety Integrations.
Learn More
EDC Migrator
Features in this section are new features for Veeva EDC Migrator.
Study Decomissioning & Database Clean-up
Use Case
As part of Veeva’s partner agreement, Veeva is required to delete all of a customer’s DEV and UAT/TEST study data after migration is complete. This feature ensures that the data is automatically deleted, rather than depending entirely on human intervention.
Description
After a Study has finished migrating or after a release, there is no formal process in place that removes a Study’s data from the applicable databases, which can lead to performance issues. After a Study has successfully migrated and after a release, this process ensures the appropriate environments and databases are cleaned up and that the migrated study is decommissioned.
Enablement & Configuration
This feature is enabled automatically.
Feature Enablement Summary
| Feature Name | Configuration | Dependencies | Day 1 Impact to Primary Users | Users with Day 1 Visibility |
|---|---|---|---|---|
| Clinical Coding | ||||
| Display Third-Party Coding Suggestions | Coder Tools | Requires a Third-Party Integration for generating suggestions. |
|
|
| Study Administration | ||||
| Snapshots: Bulk Lock & Freeze | Data Model V2 |
|
|
|
| CDMS Billing Status Support |
|
|||
| Vault CDB | ||||
| Conditional Unblinding | CDB | Masking |
|
|
| Mask Blinded Data |
|
|||
| OpenEDC: Support for Non-Veeva EDC Studies | Support | |||
| Integrations | ||||
| Safety Integration Follow Up Processing Changes |
|
|||
| EDC Migrator | ||||
| Limited Availability: In the current release, EDC Migrator is only available to early adopter customers. Contact your Veeva Services representative for details. | ||||
| Study Decomissioning & Database Clean-up |
|
|||
Enablement Legend
- Configuration: This field lists the location(s) where configuration for this feature occurs, for example, "Studio" or "EDC Tools". "Support" indicates that this feature must be enabled by Veeva Support, and "Vault Admin" indicates that configuration must be performed by a Vault Owner in the vault's Admin area.
- Dependencies: This field lists any dependencies required to use this feature, for example, Labs or Expression Engine V2. The other columns assume that the dependencies are enabled/in use.
- Day 1 Impact to Primary Users: This feature is visible and available to one or more primary user teams (Site Users, Clinical Team, and Coders) on day 1. Otherwise, this feature is either only visible to study designers or administrator users, it requires configuration before it is visible to primary users.
- Users with Day 1 Visibility: This feature is visible to these users on day 1 if no configuration occurs.