New Features in 21R2.3
Arms & Cohorts, Study Data Extract Enhancements, and more...
Release Date: September 24, 2021
We are pleased to bring you the following new features in this week's release. See a summary of feature enablement for this release below. Information on developer features (REST API) is in the Developer Portal.
CDMS
Features in this section are changes that apply to all application areas of Vault CDMS.
EDC General Enhancements
Use Case
Users no longer need to reference the audit trail to find items previously set as SDV/DMR prior to the Review Plan update to make the items no longer required. With the Last Signed Date field, custom reports can be created to include this information for a historical view into if/when a Form was last signed. Running rules via EDC Tools now provides a more detailed explanation of the actions taken by running the selected rules.
Description
In this release, the Review tab displays SDV and DMR as Completed for Items that were previously marked as SDV or DMR complete when set to either “Required” or “Optional” on the Review Plan, but were later updated to “Not Required.” This feature allows users to see the Items that were previously set as SDV/DMR complete without having to view the Audit Trail. If the data changes, or SDV/DMR is unset by a User, the SDV/DMR icons will no longer display and the current Review Plan Requirements will be honored.
A “Last Signed Date” field is now available as part of the Signature State object. This field is updated with the current datetime when a signature is applied and can be used in custom reports.
The output file for dynamic rules now contains the following details in EDC Tools:
- Add Form: Users can see where the Form is being added with the inclusion of Event Group label, Event Group sequence, Event label, and Form label
- Add Event: Users can see where the Event is being added with the inclusion of Event Group label and Event Group Sequence
- Add Assessment: Users can view the hierarchy of the source form by including the Event Group label, Event Group sequence, Event label, Form label, and Form sequence
Enablement & Configuration
Historical SDV is Auto-On and only available for studies on Review Rollup Version 2. Last Signed Date is Auto-On and only available for studies on Data Model 2.0. EDC Tools Rules outputs are Auto-on.
Clinical Coding
The following are new features for Coder, the clinical coding area for Vault Coder.
Batch Upversioning Enhancements
Use Case
Coder Admins can preserve the continuity of coding on Forms that aren’t being upversioned.
Description
In this release, there are several enhancements to the Batch Upversioning experience, such as a more intuitive UI regarding the Upversioning of Forms or both Forms and Synonym Lists. Vault also displays a series of dialogs to confirm Upversioning before running the job. If the Synonym List is Upversioning along with some but not all assigned Forms, Vault provides an option to assign a Synonym List to the assigned Forms that are not Upversioning.
Every time the user Upversions Forms or Synonym Lists, Vault provides an Impact Report that is available to download in the email confirmation once Upversioning is complete. Coder Admins have the option to delete a Synonym List.
Enablement & Configuration
These changes apply automatically.
JDrug Suggestions & Upversioning
Use Case
Customers have access to more features when coding with JDrug.
Description
With this release, users are able to Upversion the JDrug dictionary to newer releases of JDrug. Users are also able to view an Impact Report, use the Propagate Code feature, and import/export a JDrug Synonym List.
Enablement & Configuration
These changes apply automatically.
Study Design & Configuration
Features in this area apply to Studio, the study design and configuration area for Vault EDC.
Allow Studio Users to Create Organizations, Collections & Studies
Use Case
Customer organizations can now have their study designers and librarians create their own Organizations and Studies instead of relying on Veeva Services or a Vault Owner to do so.
Description
With this release, we now allow users with access to all studies (Grant Access to All Studies set to Yes) and the Design Study permission (for Studies) or Design Library permission (for Collections) to create new Organizations, Collections, and Studies in their vaults. Prior to this release, organizations had to rely on either Veeva Services or a Vault Owner to create these for them.
Enablement & Configuration
This change applies automatically, with no additional configuration required.
Arms & Cohorts
Use Case
This feature allows organizations to track changes and assignments to arms, cohorts, and substudies for managing study progression.
Description
An organization can now design a Study with any combination of Arms, Cohorts, and Substudies. Vault uses rules, with the Assign Subject Latest Arm, Assign Subject Latest Cohort, and Assign Subject Latest Substudy action types, to assign a subject to a given group. A subject can be in any combination of Arm, Cohort, and Substudy, but a subject can’t be in two or more groups of the same type at the same time. For example, a subject can be in Arm A and Cohort 2, but a subject could not be in Arm A and Arm B.
Subject Groups are subject to casebook versioning. Vault automatically references the most recent version of the Subject Group when assigning it to a subject.
This feature adds three new Casebook Variables for use when writing rules: Arm, Cohort, and Substudy.
Vault displays the Subject Groups assigned to the subject in the casebook header. Site users can hover over the Info () to view it.
Enablement & Configuration
This feature is automatically enabled for all Studies using version 2 of the expression grammar. However, to use this feature, a study designer must configure Subject Groups and rules to assign subjects to those groups.
New Subject Statuses to Align with CTMS
Use Case
This feature aligns CTMS and CDMS products, providing more value to customers and more detailed tracking of subject status in a study.
Description
In this release, we’ve added new subject statuses to align with Vault CTMS. These statuses are available for use in CDMS and will export on the SYS_SUB dataset in the study data extract. The new statuses include the following:
- Consented
- Started Treatment
- Started Follow Up
- Lost to Follow Up
Enablement & Configuration
These statuses are available automatically.
New Aggregate Functions
Use Case
This feature provides more flexibility when writing rules with aggregates.
Description
With this release, the following functions are now available for use with aggregate identifiers:
IsAnyBlank(identifier, identifier, ...): This function returnstrueif any of the values for the identifiers specified is blank.Count(identifier identifier, ...): This function returns the number of values for an aggregate identifier.CountIf(value, identifier, identifier): This function returns the number of times the specified value can be found across all of the identifiers specified.NoBlanks(identifier, identifier, ...): This function removes any blank values from an aggregate identifier.AllEqual(identifier, identifier, ...): This function returnstrueif all of the values for the specified identifiers are equal.GetAllMatches(value, identifier, identifier): This function returns an array of values from the second identifier, filtered by the Sequence Numbers where the specified value can be found in the first identifier.
As part of this feature, aggregate identifiers now support boolean and time item types.
Enablement & Configuration
These functions are automatically available in Studies using version 2 of the expression grammar.
Previous & Next for Repeating Event Groups, Forms & Item Groups
Use Case
The ability to reference the previous or next instance of a repeating object makes it easier to write rules, as users don’t need to specifically list sequence numbers with the identifiers in the rule expression.
Description
Studio now supports using [-1] (previous) and [+1] (next) to indicate that an identifier should resolve to the previous or next value of that identifier, for repeating Event Groups, Forms, and Item Groups. Previous and next identifiers require the use of an “anchor” identifier in the expression. An anchor identifier matches the previous/next identifier, but it doesn’t include the [-/+1]. Vault uses the anchor identifier as a reference to know where to calculate the previous or next instance from.
Enablement & Configuration
This feature is automatically available in Studies using version 2 of the expression grammar.
Rule Syntax Enhancements
Description
This release includes the following enhancements to rule syntax:
- For Send Email rules, Vault now supports the
has_value_changed__vattribute on the Item identifier. This attribute returnstrueif the value of the identified Item has changed since the previous form submission. - Vault now supports the
submit_counter__vattribute on@Formidentifiers and fully-qualified form identifiers. This attribute returns the number of times the identified Form has been submitted. - Vault now supports the
intentionally_left_blank__vandchange_reason__vattributes on fully-qualified identifiers. Theintentionally_left_blank__vattribute returnstrueif the identifier was marked as intentionally left blank. Thechange_reason__vattribute returns the Change Reason applied to the most recent modification of the identifier.
Enablement & Configuration
These functions are automatically available in Studies using version 2 of the expression grammar.
Study Administration
Features in this section apply to EDC Tools, a study-level administration area for Vault EDC.
Study Data Extracts: Add Additional Columns to System Datasets
Use Case
This feature provides consumers with additional data from the export to process and use for downstream analysis.
Description
In this release, additional columns are available to align with Utilities version 5. The following System Datasets will be affected:
- SYS_EVT
- SYS_FORM
- SYS_ILB
- SYS_Q
Enablement & Configuration
Available to users with access to EDC Tools and the “Manage Jobs” permission.
Study Data Extracts: General Enhancements
Use Case
These enhancements improve the consistency of the SDE columns, add additional data, and provide more information to the user in the UI about which options are available when running the SDE job.
Description
This feature provides the following general enhancements to the SDE:
- Consistency with coding column headers to remove spaces
- Included information in the Job History grid about which SDE version and options were selected
- Consistency between the Key clinical data columns and System dataset column naming
- Adding arms and cohorts fields to the SYS_SUB dataset
Enablement & Configuration
Available to users with access to EDC Tools and the “Manage Jobs” permission.
SDE: Versioning
Use Case
Versioning allows users to maintain backward compatibility with their integrations and allows them to benefit from newer options when they upgrade to the latest version of the SDE.
Description
This feature allows users to select which version of the SDE they want to run. Each version may contain a different set of datasets, columns, or other changes that will be documented on the CDMS Help page.
Enablement & Configuration
Available to users with access to EDC Tools and the “Manage Jobs” permission.
Study Listings
Use Case
Listings that were previously available only in Utilities are now available through EDC Tools. They provide operational data for forms, events, subjects and queries.
Description
With this release, we added four (4) new study listings, which study administrators can generate using the appropriate listing’s job in EDC Tools.
These study listings include:
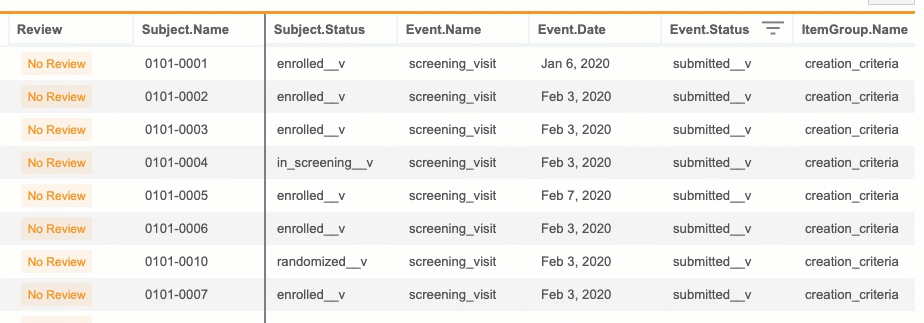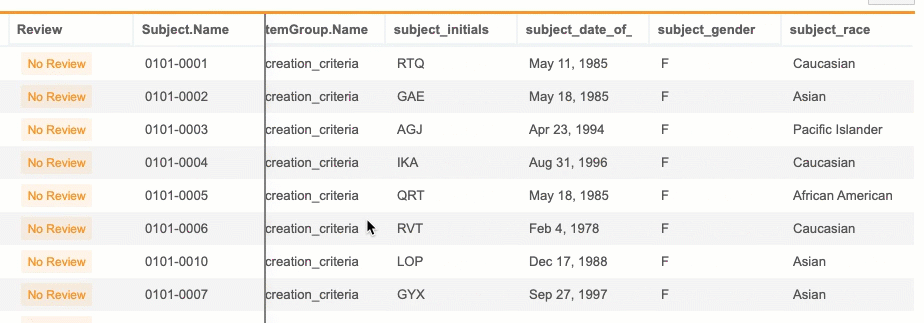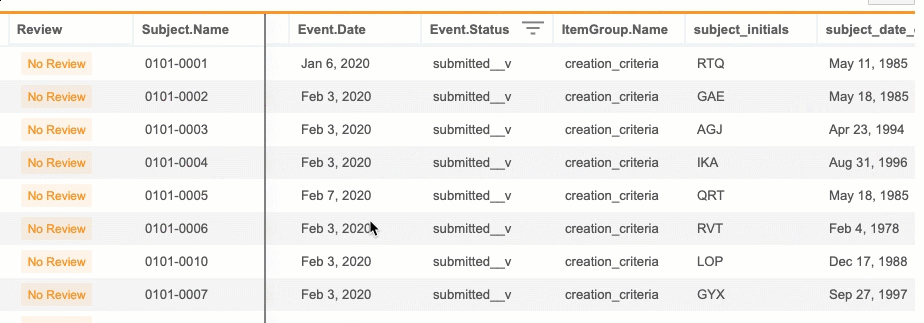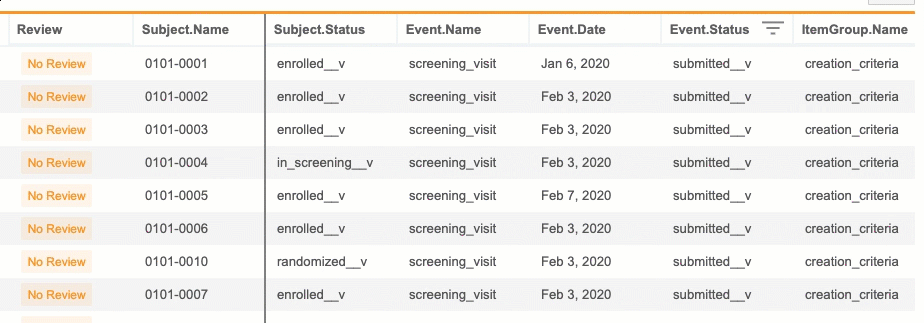
- Subject Progress Listing: Generates a list of all Subjects in a Study with status details and operational information
- Event Progress Listing: Generates a list of all Events in a Study with status details and operational information
- Form Progress Listing: Generates a CSV listing all Forms within the Study with status details and operational information
- Query Detail Listing: Generates a list of all Queries in a Study with status details and operational information
Users can choose whether or not to include restricted data in the extract.
Note that if your Study is using Data Model V1, certain columns in the Event Progress Listing and Form Progress Listing CSV outputs will be blank. Contact your Veeva Services representative to discuss upgrading to Data Model V2.
Enablement & Configuration
These jobs are available automatically.
Error Console Enhancements
Description
For each error listed in the Error Console, users can now view the associated rule’s syntax and the entire error message in the Error Details dialog. They can also download the list of errors as a CSV file.
Enablement & Configuration
These enhancements apply automatically in EDC Tools > Rules > Error Console.
Deployments
Features in this section are enhancements to deployment functionality in Vault CDMS.
Validation Environments
Use Case
This feature provides a specified environment for conducting validation activities.
Description
This release introduced a new environment type, Validation. Any existing Studies using the automatic deployment model are eligible to use this environment type. A Study can have up to 2 validation environments. Deployment administrators can deploy from UAT environments to validation environments.
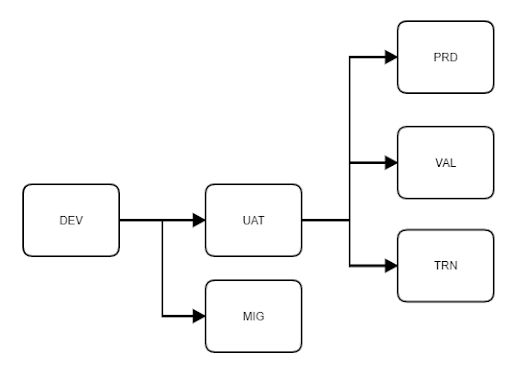
In Studio, Vault has a dedicated environment-type badge for validation environments. Study designers and librarians can use validation environments as a source copying a study or comparing casebook versions.
The following environment-level actions are available for validation environments in EDC Tools:
- Delete Study Data
- Rename
- Delete
- Lock
Enablement & Configuration
Contact Veeva Services to enable this feature for your organization.
Migration Environments
Use Case
This feature provides a specified environment-type for the migration of data from external systems into Vault CDMS.
Description
This release introduced a new environment type, Migration. Any existing Studies using the automatic deployment model are eligible to use this environment type. A Study can have up to 3 migration environments. Deployment administrators can deploy from development environments to migration environments.
In Studio, Vault has a dedicated environment-type badge for migration environments. Study designers and librarians can use migration environments as a source copying a study or comparing casebook versions.
The following environment-level actions are available for migration environments in EDC Tools:
- Delete Study Data
- Rename
- Delete
- Lock
Enablement & Configuration
Contact Veeva Services to enable this feature for your organization.
Clinical DataBase (CDB)
The following are new features for the CDB application, the Vault CDMS solution for data cleaning and reporting.
Availability: Clinical DataBase (CDB) is only available to CDB license holders. Contact your Veeva Services representative for details.
Batch Queries & Review
Use Case
Data managers can indicate and resolve issues more efficiently with batch actions.
Description
Within a listing, users can create, reply to, and close queries in batches. For review listings, users can set the status of records in bulk, including setting a new status, In Progress. All bulk actions can be applied on a per-page basis.
Enablement & Configuration
This feature is available automatically.
Review & Query Dashboards
Use Case
Actionable dashboards enable data managers to quickly identify and resolve discrepant data within a study.
Description
The new Dashboard page displays summary information for each Review Listing in the Study, including record counts and status summaries for the following review statuses:
- Not Reviewed
- In Progress
- Reviewed
- Reviewed
- Known Discrepancies
- w/ Answered Queries
- w/ Open Queries
Workbench also displays two charts:
- Outstanding Queries: Displays a donut chart of Open and Answered queries, as well as a count of queries in each Query Status
- Subjects with Outstanding Queries by Status: Displays a bar chart of outstanding queries across the Pre Screen, Randomized, In Screening, Screen Failure, Enrolled, and Withdrawn subject statuses
Enablement & Configuration
This feature is available automatically.
Pin Columns in Listings
Use Case
With large listing datasets, users will be able to pin key columns such as event, subject and subject as they review data within a Listing.
Description
Users may now specify the number of columns to be pinned to the left in a listing. This setting persists between sessions per Listing and per user.
Enablement & Configuration
This feature is available automatically.
CQL Editor Enhancements
Use Case
These enhancements provide an improved user experience for writing CQL syntax and debugging errors.
Description
CDB’s CQL Editor now supports line numbering and syntax highlighting. Functions, operators, comments, strings denoted by single quotes, integers, and header context have their own distinct highlighting. Brace matching is also supported. When the opening parenthesis is entered, the ending parenthesis autopopulates. If one side of the parenthesis is missing, the lone side is highlighted in red when selected. When one side of a parenthesis is selected, the matching parenthesis will highlight. Additionally, users can now resize the CQL Editor or open it in a new window for a larger area to write CQL in.
Enablement & Configuration
These changes apply automatically.
Role Management & Security
Features in this section are enhancements to the System Tools > Role Management and System Tools > Users areas, as well as changes to standard Study Roles, security, and access control in Vault CDMS.
Veeva Learning Integration Enhancements
Use Case
These enhancements support regulatory compliance by preventing vault-wide and study-wide users from accessing Vault CDMS before completing the appropriate training and increasing the reporting ability on the training progress of users across Studies.
Description
With this release, we made four (4) enhancements to the Veeva Learning integration.
User administrators can now assign training to users with Access to All Studies (vault-wide userse) and users with Access to All Study Environments (study-wide users). When assigning training for users with vault-wide access, Vault looks at the training requirements (Curriculum to Role Mapping) across all Studies in the vault and assigns the user those curricula in Veeva Learning (Absorb). Users with these Study Roles are required to complete the assigned courses before they can view any study-related records in CDMS. Until they have completed their training, Vault marks their Study Access as Disabled. Once training is complete, Vault updates their Study Access to Enabled. Vault uses this same logic to users who are granted access to all Environments in the Study, looking at the training requirements across all Environments in the Study.
When updating the Curriculum to Role Mapping for a Study Environment, users can now copy their changes to the mapping to one or more Studies. Note that this only copies the delta (the changes applied by the Save action). For example, if a user in the Cholecap study adds the Vault CDMS for Study Designers curriculum to the CDMS Librarian role, Vault would only copy the assignment of that curriculum. It would not update the curriculum mappings in the target study to be an exact match of Cholecap.
With this release, you can now generate the training report across one or more Studies from System Tools > Users. This training report is the same report that is available from EDC Tools > Learning Systems, except it can cover multiple Studies. The report lists a user’s training progress, including their assigned curricula, the Completion Date of each curriculum.
We updated the Training Report to include these columns:
- Last Name
- First Name
- Company
- Absorb Username (New)
- Vault Username (Relabeled, formerly “Username”
- User Status (New)
- Study
- Study Role
- Study Access
- Site Access
- Country Access (New)
- Training Required (New)
- Training Status (New)
- Date Assigned to Study (New)
- Last Modified Date (New)
- Curriculums Assigned (Relabeled, formerly “Curriculum Name”)
- Courses Assigned (New)
- Training Completion Date (Relabeled, formerly “Curriculum Completion Date”)
Enablement & Configuration
This feature is automatically enabled in any Studies where the Veeva Learning integration is enabled.
Study Role Enhancements
Description
With this release, we made the following functional enhancements to standard Study Roles and their permissions:
- In support of future enhancements, we added the Browse View permission and assigned it to the CDMS Data Manager, CDMS Lead Data Manager, and CDMS Super User roles.
- In support of future enhancements, we added the following permissions and assigned them to the CDMS Super User role:
- Browse View
- Create View
- Modify View
- Delete View
- Manage Key Mappings
- The CDMS Clinical Research Associate role now has the View Casebook permission.
- We renamed Manage Study Deployments to Manage Deployments.
- All standard and user defined roles that have the Manage Randomization now need access to all Sites to perform those functions.
Enablement & Configuration
These changes apply automaticaly to standard Study Roles.
Integrations
Features in this section are new integrations with Vault CDMS or enhancements to existing integrations.
Safety Link Support for External Labs
Use Case
Safety administrators can transfer lab data from CDMS to the safety system.
Description
With this release, users can map an External Labs form for inclusion in the E2B from Safety Configuration. If an instance of the External Labs form is linked to the Serious Adverse Event form, then the Safety Link will generate a follow-up message to the safety system. The transmitted E2B XML will include item values from the lab form.
Enablement & Configuration
A safety administrator must map an External Labs form from EDC Tools > Safety Configuration.
Coding Values in E2B
Use Case
Safety administrators can now transfer coding values from CDMS to their safety system based on the E2B ICH Guidelines.
Description
With this release, Vault automatically includes the coding values assigned in Vault Coder to an SAE in the E2B transfer to the safety system. If the coding values are later changed, Vault generates a follow up message to the safety system.
Enablement & Configuration
This change to the E2B transfer applies automatically when coding is enabled for the safety-enabled Serious Adverse Event form.
Treatment Forms in E2B
Use Case
Safety administrators can transfer more data (Treatment Forms) from CDMS to their safety system.
Description
With this release, safety administrators can configure their Study to send any linked Treatment Forms with the Safety Case to the safety system. If a Treatment Form of each Treatment Form Definition is marked as Administered to Subject and the Start Date is before the SAE’s Start Date, then Vault includes it in the Safety Case.
A newly submitted Treatment Form that meets the above criteria will generate a follow up message to the safety system and the transmitted E2B XML file will include the Item values for the Form.
Enablement & Configuration
A safety administrator must map each Treatment Form Definition from EDC Tools > Safety Configuration for Vault to include it in Safety Cases.
Long Narratives
Use Case
With long narratives, Safety Link doesn’t limit the Narrative field length and transfers all of its contents to the safety system based on the E2B ICH Guidelines.
Description
With this release, CRCs can enter up to 100,000 characters for the Case Narrative Including Clinical Course, Therapeutic Measures, Outcome and Additional Relevant Information (E2B ID H.1) field and 20,000 characters for the Reporter’s Comments (E2B ID H.2) field on the SAE form. Vault then includes the entirety of these field values in the XML file for transfer to the safety system.
Note that the narrative Item must be within a repeating Item Group.
Enablement & Configuration
The character limit changes apply automatically.
Feature Enablement Summary
| Feature Name | Configuration | Dependencies | Day 1 Impact to Primary Users | Users with Day 1 Visibility |
|---|---|---|---|---|
| CDMS | ||||
| Clinical Coding | ||||
| Batch Upversioning Enhancements |
|
|||
| JDrug Suggestions & Upversioning |
|
|||
| Study Administration | ||||
| Study Data Extracts: Add Additional Columns to System Datasets |
|
|||
| SDE: Versioning |
|
|||
| Study Listings |
|
|||
| Error Console Enhancements |
|
|||
| Study Design & Configuration | ||||
| Allow Studio Users to Create Organizations, Collections & Studies |
|
|||
| Arms & Cohorts | Studio | Expression Grammar V2 |
|
|
| New Subject Statuses to Align with CTMS |
|
|||
| New Aggregate Functions | Expression Grammar V2 |
|
||
| Previous & Next for Repeating Event Groups, Forms & Item Groups | Expression Grammar V2 |
|
||
| Rule Syntax Enhancements | Expression Grammar V2 |
|
||
| Deployments | ||||
| Migration Environments |
|
|||
| Integrations | ||||
| Safety Link Support for External Labs | EDC Tools |
|
||
| Coding Values in E2B |
|
|||
| Treatment Forms in E2B | EDC Tools |
|
||
| Long Narratives |
|
|||
| Role Management & Security | ||||
| Veeva Learning Integration Enhancements | Veeva Learning Integration |
|
||
| Vault CDB | ||||
| Batch Queries & Review |
|
|||
| Review & Query Dashboards |
|
|||
| Pin Columns in Listings |
|
|||
| CQL Editor Enhancements |
|
|||
Enablement Legend
- Configuration: This field lists the location(s) where configuration for this feature occurs, for example, "Studio" or "EDC Tools". "Support" indicates that this feature must be enabled by Veeva Support, and "Vault Admin" indicates that configuration must be performed by a Vault Owner in the vault's Admin area.
- Dependencies: This field lists any dependencies required to use this feature, for example, Labs or Expression Engine V2. The other columns assume that the dependencies are enabled/in use.
- Day 1 Impact to Primary Users: This feature is visible and available to one or more primary user teams (Site Users, Clinical Team, and Coders) on day 1. Otherwise, this feature is either only visible to study designers or administrator users, it requires configuration before it is visible to primary users.
- Users with Day 1 Visibility: This feature is visible to these users on day 1 if no configuration occurs.