New Features in 19R3.5
Progressive Display, Send Email Rules, and more...
Release Date: March 13, 2020
We are pleased to bring you the following new features in this week's release. See a summary of feature enablement for this release below. Information on developer features (REST API) is in the Developer Portal.
Data Entry
Features in this section are changes to the Data Entry tab, a working area for investigators and clinical research coordinators to enter study execution data.
Data Entry Navigation Enhancements
Description
In this release, the Common Forms section can be expanded and collapsed using the More and Less buttons.
Any Studies created after this release automatically have version 2 of the Data Entry UI enabled.
Enablement & Configuration
This change applies automatically to any Studies using version 2 of the the Data Entry UI.
Display Item Label Above Control for Indented Items
Use Case
This provides better visibility on indented Items for data entry users.
Description
With this release, Vault now displays the item’s Label above the item’s control for any Items that are indented.
Enablement & Configuration
This change applies automatically, with no additional configuration required for Items that are already indented.
Data Review
Features in this section are changes to the Review tab, a working area for clinical research associates and data managers, or to review functionality within the Data Entry tab.
Prevent Recreation of Manually Closed System Queries
Use Case
This provides a better experience for site users so they don’t have to keep addressing the same query multiple times.
Description
System queries that have been closed will not be recreated if the form is resubmitted and the Item values that are being evaluated in the rule have not changed. If any of the items in the rule have been modified since the query was closed and the form is resubmitted, the rule logic will be executed again and a query will be created if the rule expression is met.
Enablement & Configuration
This change applies automatically with no additional configuration required.
Vault Coder
The following are new features for the Vault Coder application, the Vault CDMS solution for clinical coding.
Automating the Autocoding and Suggestions Job
Use Case
Coders will have the latest data about Code Requests.
Description
Because autocoding is now an active and automated process, a Coder may be halfway through coding a list of pending Code Requests when those records are autocoded in the background because a matching record was approved or added to the assigned Synonym List. When records are autocoded, users will see an out-of-sync notification at the top of the coding page. This notification informs the user that records have been autocoded and must be refreshed to retrieve the latest data. Clicking the Refresh button will update any relevant records and preserve the Coding Status and Query Status filters if any are applied.
With this release, we have removed the Autoassign button, as users no longer have to wait for the Autocoding job before records are autocoded.
Enablement & Configuration
This feature is automatically available in Coder.
Automating the Autocoding and Suggestions Job
Use Case
Automatic autocoding and suggestions keeps data consistently updated without Vault having to run unnecessary jobs.
Description
In this release, the Autocoding and Suggestions job has been changed to an active and automated process that runs throughout the day, providing the user with Autocoding and Suggestions that are constantly up-to-date with evolving Synonym Lists. Autocoding jobs can no longer be scheduled and any currently scheduled Autocoding jobs will be cancelled with this release. Because autocoding is now an active and automated process, a Coder may be halfway through coding a list of pending Code Requests when those records are autocoded in the background because a matching record was approved or added to the assigned Synonym List. When records are autocoded, users will see an out-of-sync notification at the top of the coding page. This notification informs the user that records have been autocoded and must be refreshed to retrieve the latest data. Clicking the Refresh button will update any relevant records and preserve the Coding Status and Query Status filters if any are applied. The Autoassign button has been removed, as users no longer have to wait for the Autocoding job before records are autocoded.
Enablement & Configuration
This feature is automatically available in Coder.
Study Design & Configuration
Features in this area apply to Studio, the study design and configuration area for Vault EDC.
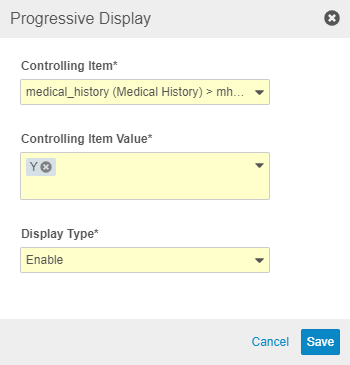
Progressive Display
Use Case
Progressive display allows for more intuitive form design to support data entry users, leading to improved data quality. For example, a form with thirty total data items can display only the relevant items for the subject an investigator is collecting data for.
Description
With this release, study designers can design a form such that data collection items are dynamically displayed or hidden based on user-entered data. This enhancement allows study designers to hide unneeded items instead of simply disabling them, streamlining the data entry experience and allowing investigators to focus only on items that need to be addressed.
To progressively display items, study designers can set the new Progressive Display properties, Controlling Item, Controlling Item Value, and Display Type on their forms from Studio > Schedule.
In support of this enhancement, we’ve added items for progressive display configuration to the Study Design Specification and annotated PDF.
Enablement & Configuration
Progressive Display is only available in studies using the automated deployment model and version 2 of the expression grammar. To enable the Progressive Display feature for existing studies, a member of the Veeva Services team must set the Rule Version field to “2” on the Study Configuration object record for a study. That change exposes the configuration options for Progressive Display within Studio. A study designer must still create a new version and configure Items for progressive display for this feature to be visible to data entry users.
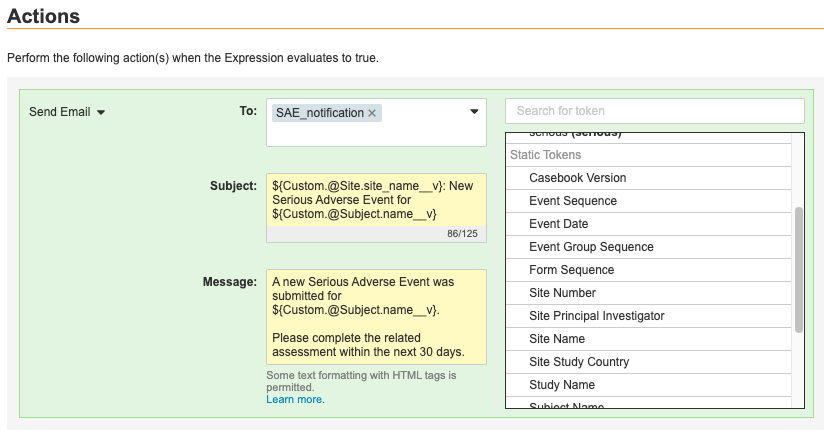
Send Email Rule Configuration
Use Case
The Send Email rule action was released in 19R3, but it was only configurable via Business Admin. This feature provides a UI for the configuration in Studio and EDC Tools.
Description
With this release, we added the ability to fully configure Send Email rules in Studio. Study designers can create an Email Group and create a Rule from Studio, without the need to access Admin > Business Admin. The Rule Editor includes a Message Template Editor, where users can create template email messages with text, static and dynamic tokens, and some HTML formatting.
To support Send Email rules, we added the ability to assign Users to Email Groups from the new tab, EDC Tools > Email Group Assignment. A lead data manager can add users from the current Study and from other study environments.
As part of this feature, we added a new permission, Manage Email Group Assignment, which controls the ability to assign users to Email Groups. This permission is assigned to the CDMS Lead Data Manager role by default.
Enablement & Configuration
These changes apply automatically in vaults where Role by Study is enabled. This feature is only available in studies using the automated deployments model. In those studies, these options display automatically in Studio and EDC Tools, but an organization must configure Send Email rules and Email Groups as part of their study.
Form Linking & Copy Indication from Link
Use Case
The Copy Indication from Link feature serves as a shortcut to link Form Records and, more importantly, to use an entered Adverse Event Symptom or Medical History condition as the Indication value on a Concomitant Medications form.
Description
With this release, users can configure and associate related forms through Form Linking in the Data Entry and Studio tabs. For example, one or more Concomitant Medication forms can be linked to an Adverse Event or Medical History form so as to group similar or related data or indicate cause and effect.
Users will be able to list all records that should be linked for a given subject when creating a link. Users can also filter the list of related form records by selecting records in the same event as the current form or by viewing all records across different events, if applicable. The “Show Selected” filter also allows the user to filter records by selected records. Users will also be able to use the search box filter to narrow down specific related records. The search box filter will only search on columns where the value is entered into a free item control so that data values from the codelist item control will not be searchable from the search box filter. Form links can be created in the Studio tab in the Form Links section.
Once a Form Link has been established, users can leverage that link to set Indication values for a Form in the coding configuration. If a WHODrug-based form has an Indication item, that item can be further configured to retrieve its value from a linked form. For example, an Adverse Event or Medical History form can be selected to have its respective Symptom or Condition serve as the Indication on a Concomitant Medications form. If that Form Instance was not previously established as a Link Instance, then it will become established as one. Site users will still have the option to enter free text into the Indication Item field if there aren’t any relevant Adverse Events or Medical History symptoms or conditions to use.
Enablement & Configuration
A study designer must add Form Links to a study design before this feature is visible to data entry users. This feature is only available in Studies using the automated deployment model and version 2 of the expression grammar.
ODM Import & Export for Automated Deployment Studies
Use Case
This enhancement provides a method to take in reusable and standard study designs via ODM, allowing for sponsors to more easily adopt Vault CDMS when they have established study designs.
Description
With this release, we added the ability to export and import Veeva Extended ODM files in Studio for Studies using the automated deployment model. Export is now available throughout the study development lifecycle, but Import is limited to the first version of the Casebook Definition. Import automatically ignores the casebook version of the file, importing the file in as version 1. The Publish action remains unavailable, as Vault publishes casebook versions during deployment to production for automated deployment studies.
There will be no additional enhancements to the Veeva Extended ODM file except for when new features would cause conflicts for manual deployment Studies.
Enablement & Configuration
This feature is available automatically in all Studies using the automated deployment model.
Create #define Statement from Item Selector
Use Case
This features allows study designers to quickly create #define statements for Items.
Description
With this release, study designers can now add an Item identifier in as a #define statement from the Item Selector. Statements created from the This Form option use an @Form variable, while statements created from the All Forms option use a fully-qualified identifier.
Enablement & Configuration
This feature is automatically available in Studio for studies using v2 of the expression grammar.
Reciprocal Rules
Use Case
This feature removes the need to create multiple Rules when comparing fully-qualified object (an Item or Event Date) and an Item on a Form that is used in multiple locations on the schedule.
Description
Any rules created after this release with wildcard Form identifiers (@Form) are now “reciprocal”. This means that rules firing from a fully-qualified identifier now evaluate every instance of that Form on the study schedule. Prior to this release, those rules would not fire in that direction and would only work from @Form towards a fully-qualified object.
Rules created prior to this release will continue to work the same. Customers can work with Veeva Services to switch existing Rules to the new, reciprocal behavior if needed.
Enablement & Configuration
This change applies automatically to Studies using version 2 of the expression grammar.
Deployments
Features in this section are enhancements to deployment functionality in Vault CDMS.
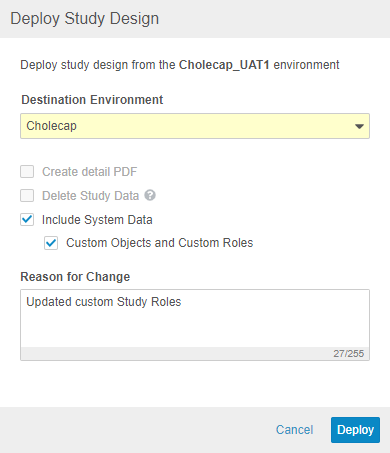
Automated Deployments Include Custom Study Roles & Objects
Use Case
This enhancement removes the need to manually reconfigure custom Study Roles and custom objects in each environment.
Description
With this release, Vault supports deployment of custom Vault objects and custom Study Roles as part of study deployments. Any custom objects and custom Study Roles that are defined in the Deployment Whitelist will be included in deployments from environment to environment. To exclude a custom role from a deployment, it must be deleted from the source environment. Vault also includes these custom roles and objects when restoring an environment. To deploy a study, a user needs the CDMS Deployment Administrator or EDC Deployment Administrator study roles. In this release, we only support the ability to deploy custom vault objects and custom study roles along with a study design deployment.
Enablement & Configuration
To deploy custom Study Roles and the configuration for custom Vault objects, work with Veeva Services to add those items to the Deployment Whitelist. After that configuration is complete, select Include System Data when deploying to an environment in a different vault to deploy custom Study Roles and Vault objects. This feature is only available in automated deployment studies.
Role Management & Security
Features in this section are enhancements to the System Tools > Role Management and System Tools > Users areas, as well as changes to standard Study Roles, security, and access control in Vault CDMS.
Study Designer Role Enhancements
Use Case
A user no longer needs to have the Vault Owner security profile to design their study.
Description
With this release, we’ve updated the existing CDMS Study Designer role and added the CDMS Study Designer Read Only role. The CDMS Study Designer role is intended for use within non-production environments. This role has complete access to Studio, the Data Entry tab, EDC Tools > Sites, and EDC Tools > Countries for creating and testing their study design. In production environments, these users are meant to have the CDMS Study Designer Read Only role, which provides read-only access to Studio, but no access to other application areas. To support this change, we’ve enabled object lifecycles and matching sharing rules (dynamic access control) on all design definition objects.
As part of this enhancement, the CDMS Study Designer no longer has the ability to create a new Study Master from Studio. This task must now be performed by a user with the Vault Owner security profile.
In 19R3, we released the initial version of the CDMS Study Designer role, but that role was supported only by a security profile and didn’t allow complete access control on a study-by-study basis. With this release, both study designer roles allow for the same granularity of access control as any other standard Study Role.
Enablement & Configuration
These changes apply automatically in vaults where Role by Study is enabled.
New "Manage Study Roles" Permission
Use Case
A user no longer needs to have the Vault Owner security profile to design their study.
Description
With this release, we added a new permission, Manage Study Roles. This permission controls the ability to create, edit, and delete custom Study Roles from Tools > Role Management. By default, this permission is assigned to the CDMS Lead Data Manager and CDMS User Administrator standard study roles.
Enablement & Configuration
This new Manage Study Roles permission is automatically added to any vaults where Role by Study is enabled.
Include Standard Roles in Roles & Permissions Matrix Download
Use Case
This enhancement allows for easy documentation of an organization’s Study Role permissions.
Description
Users with access to Tools > Role Management can now download a report of which permissions are assigned to standard study roles in addition to the custom roles. Users can now download into excel all the permissions that make up the study roles.
Enablement & Configuration
This feature is automatically available in vaults where Role by Study is enabled.
Integrations
Features in this section are new integrations with Vault CDMS or enhancements to existing integrations.
Vault EDC/CTMS Connection with Spark
Use Case
By connecting Vault EDC and CTMS via Spark and HTTP callout, customers can:
- Have confidence that both applications are working in unison
- Receive and update Subject data in near-real time
- Remove the manual effort of creating the same records in two places
- Eliminate the need for manual, expensive, and time-consuming integrations between EDC and CTMS
Description
This feature allows customers who own both the Vault EDC and Vault CTMS applications to connect those Vaults together for the purpose of transferring Studies, Study Countries, and Sites from Vault CTMS to Vault EDC and Subjects, Events, and Event Definitions from EDC to CTMS using Spark. This replaces the current process of sending Subjects and Events via FTP from Vault EDC to Vault CTMS. Vault sends Spark messages between the two applications whenever there is a data change. Upon feature enablement and activation of the Connection, Vault sends all Subjects, Events, and Event Definitions from EDC to CTMS.
Enablement & Configuration
Contact Veeva Support to enable this feature in your vault. This feature is only available to customers using both Vault EDC and Vault CTMS.
Feature Enablement Summary
| Feature Name | Enablement | Application |
|---|---|---|
| Data Entry | ||
| Data Entry Navigation Enhancements | Auto-on * In Studies using version 2 of the Data Entry UI |
EDC |
| Display Item Label Above Control for Indented Items | Auto-on | EDC |
| Data Review | ||
| Prevent Recreation of Manually Closed System Queries | Auto-on | EDC |
| Vault Coder | ||
| Automating the Autocoding and Suggestions Job | Auto-on | Coder |
| Automating the Autocoding and Suggestions Job | Auto-on | Coder |
| Study Design & Configuration | ||
| Progressive Display | Study Feature Flag * In studies using automated deployments and version 2 of the expression grammar. Note that this feature is enabled By Study Build in studies created after the 20R1 release. |
EDC |
| Send Email Rule Configuration | By Study Build * In studies using the automated deployment model and version 2 of the expression grammar |
EDC |
| Form Linking & Copy Indication from Link | By Study Build * In studies using the automated deployment model and version 2 of the expression grammar |
EDC |
| ODM Import & Export for Automated Deployment Studies | Auto-on * In studies using the automated deployment model |
EDC |
| Create #define Statement from Item Selector | Auto-on * In studies using version 2 of the expression grammar |
EDC |
| Reciprocal Rules | Auto-on * In studies using version 2 of the expression grammar |
EDC |
| Role Management & Security | ||
| Study Designer Role Enhancements | Auto-on * In vaults where Role by Study is enabled |
All |
| New "Manage Study Roles" Permission | Auto-on * In vaults where Role by Study is enabled |
All |
| Include Standard Roles in Roles & Permissions Matrix Download | Auto-on * In vaults where Role by Study is enabled |
All |
| Deployments | ||
| Automated Deployments Include Custom Study Roles & Objects | Auto-on * The enw options for deployments display automatically to deployment administrators, but a Vault Owner must first create Deployment Whitelist records for those options to be useable. |
All |
| Integrations | ||
| Vault EDC/CTMS Connection with Spark | Support | EDC, CTMS |
Enablement Legend
| Enablement | Description |
|---|---|
| Auto-on | Automatically activated and no configuration is required before using the feature; note that in some cases, a new feature is dependent on another feature that must be enabled or configured. |
| Study Feature Flag | This feature is available by configuration within the Study Configuration object (or similar). To enable a feature using study configuration, navigate to Admin > Business Admin > Study Configuration and edit the Study Configuration record for your Study. |
| By Study Build | The configuration options for this feature are available automatically in Studio, EDC Tools, Coder Tools, or System Tools, but you must configure them within your Study for those options to apply. |
| Support | On/off option controlled by Support. |