New Features in 19R3.4
User Activity Report, Ability to Create Production Environments, and more...
Release Date: February 18, 2020
We are pleased to bring you the following new features in this week's release. See a summary of feature enablement for this release below. Information on developer features (REST API) is in the Developer Portal.
Data Entry
Features in this section are changes to the Data Entry tab, a working area for investigators and clinical research coordinators to enter study execution data.
Allow Deletion of References in Signature Binding
Use Case
Customers will not have to rely on Veeva to delete forms or event dates that have been signed.
Description
With this release, Vault will be able to delete signed Forms and Event Dates. Prior to 20R1, signed Forms and Event Dates required a change control request to remove due to signature binding records. Users can now delete signed Forms and Event Dates without a change control request. If there are any signature bindings related to form or event date records that need to be deleted, any signature binding records that reference those records will be deleted first, thus allowing the user to delete the Form or Event Date record.
Enablement & Configuration
This feature is automatically available in the Data Entry tab.
Data Entry Navigation Enhancements
Use Case
The updated UI will help Data Entry users to more efficiently perform their activities. Changes and updates were made based on site user feedback.
Description
In this release, the Casebook Schedule page has been combined with the Form Display page to create a better navigational experience for the user. All Events and Forms in the Study Schedule are now displayed next to the Form panel, allowing Data Entry users to navigate to any Form within any Event. Any activities previously performed by CRAs or DMs (SDV, DMR, Freeze, Lock, etc.) will now need to be performed in the Review tab.
Enablement & Configuration
Organizations may enable this feature by updating the Data Entry Version field on their study’s Study Configuration object record.
Vault Coder
The following are new features for the Vault Coder application, the Vault CDMS solution for clinical coding.
Automating the Autocoding and Suggestions Job
Use Case
Automatic autocoding and suggestions will keep data consistently updated without Vault having to run unnecessary jobs.
Description
In this release, the Autocoding and Suggestions job has been changed to an active and automated process that runs throughout the day, providing the user with Autocoding and Suggestions that are constantly up-to-date with evolving Synonym Lists. Autocoding jobs can no longer be scheduled and any currently scheduled Autocoding jobs will be cancelled with this release.
Enablement & Configuration
This feature is automatically available in Coder.
Study Design & Configuration
Features in this area apply to Studio, the study design and configuration area for Vault EDC.
Copy Rules from Another Study
Use Case
Copying rules allows users to make better use of previously defined rules from other Studies in their vault. This speeds study development by reducing the effort to write new rules that are based on common logic that has already been written.
Description
Users can now copy rules from other studies in the same manner that they can copy Event Groups, Events, and Forms. Rules can be copied even when they can not reconcile with objects in the study design. These rules are considered broken, or invalid, and users can easily identify them using the Invalid indicator in the browse view (Studio > Rules) and in the Rule Editor.
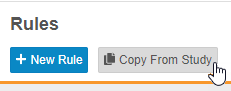
Enablement & Configuration
This feature is automatically available in Studio.
Optional Requirement Mode Support in the Review Plan Editor
Use Case
This feature removes the need to configure the Optional Requirement Mode through Business Admin.
Description
In this release, the Review Plan Editor has been enhanced to support the Optional Requirement Mode for Event Dates and Items. The Optional Requirement mode can be chosen at the Form level – Form by Form or in bulk – and at the Item Group/Item level.
Enablement & Configuration
This feature is automatically available in Studio, but a study designer must update a Review Plan to include optional review items for this change to be visible to CRAs and data managers in the Review tab.
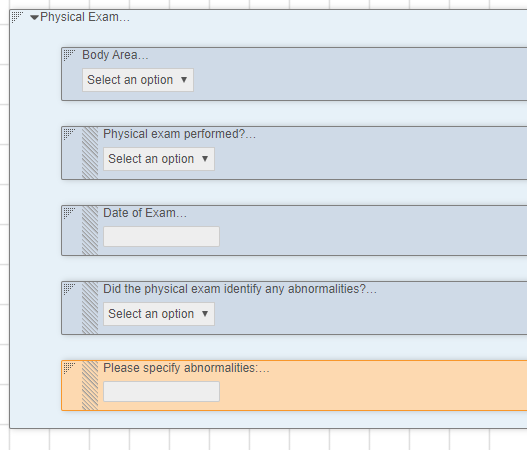
Item Indentation
Use Case
The ability to indent items provides more design options for form layout. Study designers can indent items to display items as a nested list and organize items hierarchically.
Description
Study designers can now indent Items within a Form. Each Item can be indented up to 3 levels. Indentation is available for both single and double column layouts. For composite rows, the row’s indentation level depends on the first (leftmost) item in the composite row.
This feature introduces a new Item Definition property, Indent Level. Study designers can use this property to set an item’s indent level. Studio show’s an item’s indent level with the Indent Level Indicator.
Enablement & Configuration
This feature is available by study build in all CDMS vaults. The configuration options for item indentation are available automatically in Studio, and Study Designers can introduce it in their next casebook version right away.
New Functions in the Formula Expression Grammar
Use Case
Study designers can create rules using these new functions. This feature supports future enhancements surrounding rules that reference repeating objects.
Description
This feature introduces the following new functions:
Power(): Calculates the value of a number raised to the power of another number (exponent)Sum(): Calculates the sum of the provided numbersAverage(): Calculates the average of the provided numbersStartOfDay(): Returns a DateTime in UTC that is equivalent of the beginning of the day in the specified timezone
The Today() function now also accepts an optional timezone parameter.
Note: Sum(), Average(), Min(), and Max() do not yet support providing an array of values as an argument. Providing an Item from a repeating item group does not apply the function for all the values of that item across that item group.
Enablement & Configuration
These functions are automatically available for use in studies using version 2 of the expression grammar. Study designers can begin creating rules using them right away.
Rule Editor Enhancements
Use Case
These enhancements ease rule creation, eliminating the need to rely on the Study Design Specification to know identifier paths when creating variables.
Description
With this release, we made the following enhancements to the Rule Editor:
- Users can search for Item identifiers to reference in their rule expression. They can limit the search to the current form (selected in the Rule Details panel) or search across all forms in the Study.
- Users can collapse and expand the Rule Details panel.
Enablement & Configuration
This feature is automatically available in the Rule Editor (version 2 of the expression grammar).
Prevent Creation of First Instance for Repeating Forms Added by an Add Form or Add Event Rule
Use Case
This feature allows for the consistent behavior of dynamic repeating forms added by the Add Schedule, Add Event, and Add Form rule actions.
Description
With this release, Vault no longer automatically creates the first instance of a repeating Form if the Form is added by an Add Form or Add Event rule. For existing Studies that want to continue with the original behavior of adding the first instance, a Vault Administrator can set the Dynamic Repeating Form Instance field on the Study Configuration object to Yes.
Enablement & Configuration
This feature is automatically enabled for existing Studies. To return to the original behavior (creating the first instance of the Form), set the Dynamic Repeating Form Instance field to Yes.
Status Change Date for Set Subject Status Rules
Use Case
This enhancement supports the connection between CDMS and Vault CTMS, as CTMS pulls the Subject Status changes from the Subject object.
Description
Study designers can configure a Set Subject Status rule action to set the date for the status change to an Event Date or date from a date-type Item.
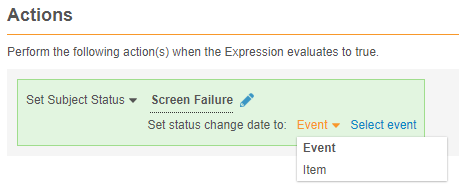
Enablement & Configuration
This feature is automatically available in Studio. Existing Set Subject Status rules will continue to work, but upon modification, a study designer must select a date to save the rule.
Select All for Assessments Item Group Visibility
Use Case
The new selection allows for more rapid configuration of Assessments, saving time and effort.
Description
With this release, users have the option to select all Items under an Item Group when setting Visibility for an Assessment Definition, in addition to the ability to select Items independently.
Enablement & Configuration
This feature is automatically available in Studio.
Study Administration
Features in this section apply to EDC Tools, a study-level administration area for Vault EDC.
User Activity Report
Use Case
With the User Activity Report, customers can track who had access to the system, thereby ensuring availability and compliance with data privacy and security regulations.
Description
The User Activity Report lists user actions and user login attempts in chronological order for a given time period. With this report, users can track various User Actions such as user logins, password resets, and users that have been created as well as Study access changes like LMS training status, study/site/country access, and study roles. If a user is associated with multiple roles or performs multiple actions, each action is listed as one row in the report. The user that runs the report will receive the finished report via email as a downloadable CSV.
Enablement & Configuration
This feature is automatically available in EDC Tools.
EDC Tools Functional Enhancements
Description
With this release, we made the following functional enhancements to EDC Tools:
- Lead data managers can now run the SDV Re-assignment and DMR Re-assignment jobs at the site level. Prior to this release, lead data managers could only run these jobs at the study level.
- Lead data managers can now create a Study Country and use it while creating a new Site. This removes the extra step of adding a Study Country to the Study before creating a Site.
- All email notifications sent from Vault CDMS now include the Vault CDMS logo.
- The Manage Users permission (in Role by Study) no longer automatically grants access to edit and delete Study Site and Study Country records. We updated the CDMS User Administrator study role to have the Manage Study Sites and Manage Study Countries permissions.
Enablement & Configuration
These enhancements are available automatically in all vaults.
Deployments
Features in this section are enhancements to deployment functionality in Vault CDMS.
Create a Production Environment from EDC Tools
Use Case
Deployment administrators can now create a production environment if one wasn’t already created for their study. Study designers can also choose to not create a production environment for studies used as design libraries.
Description
With this release, deployment administrators can now create a production-type environment (Study Instance record) from EDC Tools > Environments. Prior to this release, if a study designer didn’t create a production environment when creating the Study Master, there was no way to create a production environment. This enhancement addresses that issue.
Note that only one (1) production environment is allowed per study.
Enablement & Configuration
This feature is automatically available in studies using the automated deployment model.
Role Management & Security
Features in this section are enhancements to the System Tools > Role Management and System Tools > Users areas, as well as changes to standard Study Roles, security, and access control in Vault CDMS.
Removed Manage Study Lock Permission from CDMS Deployment Administrator
Description
With this release, we removed the Manage Study Lock permission from the CDMS Deployment Administrator standard Study Role. This role typically does not require this permission. If an organization would prefer deployment administrators to be able to lock studies, they can create a custom Study Role.
Enablement & Configuration
This change applies automatically in all vaults where Role by Study is enabled.
New "View Coding" Permission
Use Case
Customers can now create custom roles that allow users to view coding progress without impacting already coded terms.
Description
With this release, we introduced View Code, a new permission that allows users to view coding progress without impacting terms that have already been coded.
This permission is automatically assigned to the CDMS Clinical Coder, CDMS Clinical Coder Administrator, and CDMS Study Designer study roles.
Enablement & Configuration
This feature is automatically available in vaults where Role by Study is enabled.
New "Workbench Tab" Permission in Role Management
Description
With this release, we added the new Workbench Tab permission to Tools > Role Management. This permission controls the ability to access the Workbench tab and run or schedule the Workbench Export job.
Enablement & Configuration
This feature is only available in vaults that contain the Data Workbench application. Contact your Veeva Services representative for details.
Allow Deletion of Custom Study Roles
Use Case
The ability to delete unused roles allows organizations to maintain a cleaner list of Study Roles. Before deployments, a lead data manager or user administrator can delete unused Study Roles in the source environment to prevent them from being deployed to production.
Description
Prior to this release, users could not delete custom Study Roles from Tools > Role Management once a role was no longer needed. Unused Study Roles can build up over time and create confusion when assigning roles to users. With this release, users with access to Tools > Role Management can now delete custom Study Roles that don’t have any users assigned that role. Standard Study Roles (prepended with “CDMS”) remain non-editable and non-deletable.
As part of this enhancement, we renamed the Edit Role row as Manage Row and replaced the Edit button with an Actions menu. That Actions menu includes the Edit, Rename, and Delete actions.
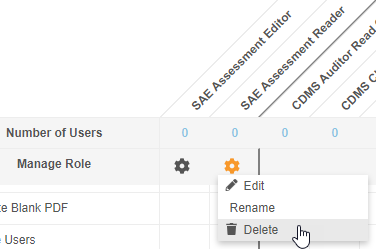
Enablement & Configuration
This change applies automatically in all vaults where Role by Study is enabled.
Manage Custom Object Permissions in Role Management
Use Case
This reduces the need for a Vault Owner to create custom sharing rules on objects.
Description
Role Management now supports setting Read, Edit, and Delete permission on custom Vault objects for custom Study Roles.
If a custom object (using the “__c” namespace) has an object reference field to the Study (study__v) object, a lifecycle, and Matching Sharing Rules enabled, and there is a record in the Deployment Whitelist for that object, Vault automatically lists it in the new Custom Objects section of the permissions table.
Vault also no longer adds newly created objects to the CDMS All Objects Read Only permission set. Instead, a Vault Owner must assign permissions on the object to the Security Profile associated with a custom Study Role.
As part of this enhancement, we renamed the Functions section of the permissions table as Permissions. With this release, the standard CDMS User Administrator study role will now be custom.
Enablement & Configuration
This feature is automatically available for vaults where Role by Study is enabled and there are custom objects configured such that they display. Before custom objects become available in Role Management, a Vault Owner must first add those objects to the Deployment Whitelist. Contact your Veeva Services representative to discuss configuration of your custom objects.
Custom Object Support in Role Management
Use Case
This reduces the need for a Vault Owner to create custom sharing rules on objects.
Description
Role Management now supports setting Read, Edit, and Delete permission on custom Vault objects for custom Study Roles.
If a custom object (using the “__c” namespace) has an object reference field to the Study (study__v) object, a lifecycle, and Matching Sharing Rules enabled, Vault automatically lists it in the new Custom Objects section of the permissions table.
Vault also no longer adds newly created objects to the CDMS All Objects Read Only permission set. Instead, a Vault Owner must assign permissions on the object to the Security Profile associated with a custom Study Role.
As part of this enhancement, we renamed the Functions section of the permissions table as Permissions.With this release, the standard CDMS User Administrator study role will now be custom.
Enablement & Configuration
This feature is automatically available for vaults where Role by Study is enabled and there are custom objects configured such that they display. Contact your Veeva Services representative to discuss configuration of your custom objects.
Download Study Role Permissions Report as an Excel™ File
Use Case
This feature is automatically available in vaults where Role by Study is enabled.
Description
Users with access to Tools > Role Management can now download an Excel™ file with a report of which permissions are assigned to which Study Role. Users can now download into excel all the permissions that make up the study roles. In this release, the report includes only custom Study Roles.

Enablement & Configuration
This enhancement allows for easy documentation of an organization’s Study Role permissions.
Provide Federated ID during User Creation (UI)
Use Case
This change allows users to provide a Federated ID for a user account when creating users manually, empowering organizations to leverage single sign-on (SSO) with Vault.
Description
When creating and editing users from EDC Tools > Users, user administrators can now provide a Federated ID. Vault uses Federated ID to associate the user’s account with an external user ID for use in single sign-on (SSO) systems and other integration purposes.
Prior to this release, user administrators could only provide a Federated ID when creating users via the API, user import, or by editing the User from Admin > Users & Groups.
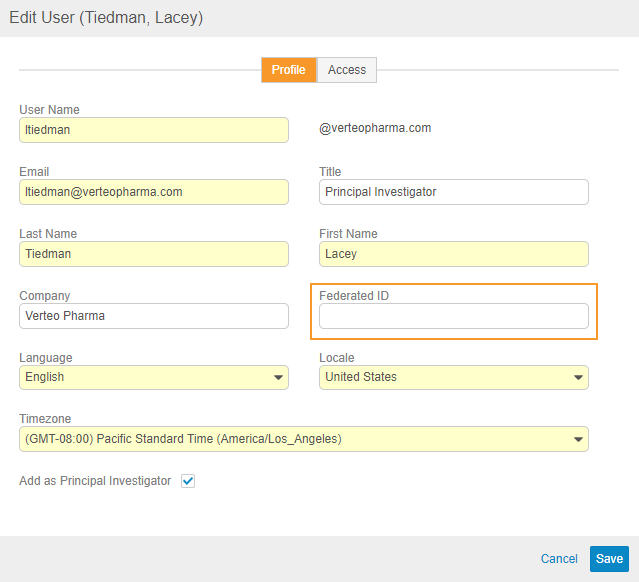
Enablement & Configuration
This feature is automatically available and visible to users with access to EDC Tools > Users.
Separate Security Profiles for Data Entry Study Roles
Use Case
Creating separate Security Profiles for each of these roles reduces confusion and eases the creation of custom Security Profiles based on these.
Description
Prior to this release, the CDMS Principal Investigator, CDMS Sub Investigator, and CDMS Clinical Research Coordinator study roles shared the Data Entry security profile. To keep these roles consistent with other Study Roles and reduce confusion when creating Groups, we created three separate security profiles for these standard roles:
- CDMS Principal Investigator
- CDMS Sub Investigator
- CDMS Clinical Research Coordinator
Enablement & Configuration
These new Security Profiles are available automatically in all vaults where Role by Study is enabled.
Integrations
Features in this section are new integrations with Vault CDMS or enhancements to existing integrations.
Veeva Learning Training Report
Use Case
Compliance teams can use this report to track the progress of user training and follow up with users whose training is incomplete.
Description
With this release, we added the ability to export a report tracking study users’ progress through their assigned training curricula. Lead data managers and user administrators can use the new Generate Training Report action to generate the report file (ZIP) from EDC Tools > Learning Systems. The report includes information about each user, including their assigned curricula and, if available, the date that the user completed their training.
Any user with the Manage Learning permission (for Role by Study) or the EDC: Study Tools: Access permission (for profile-based security) can generate this report.
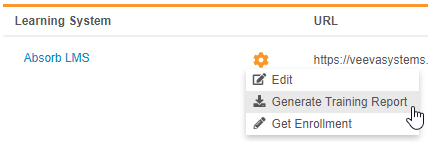
Enablement & Configuration
This feature is available automatically in studies where the Veeva Learning integration is enabled. In studies where the integration is not enabled, enable the integration to use this feature.
Feature Enablement Summary
| Feature Name | Enablement | Application |
|---|---|---|
| Data Entry | ||
| Allow Deletion of References in Signature Binding | Auto-on | EDC |
| Data Entry Navigation Enhancements | Study Feature Flag | EDC |
| Vault Coder | ||
| Automating the Autocoding and Suggestions Job | Auto-on | Coder |
| Study Design & Configuration | ||
| Copy Rules from Another Study | Auto-on | EDC |
| Optional Requirement Mode Support in the Review Plan Editor | Auto-on | EDC |
| Item Indentation | Auto-on | EDC |
| New Functions in the Formula Expression Grammar | Auto-on * In studies using version 2 of the expression grammar |
EDC |
| Rule Editor Enhancements | Auto-on * In studies using version 2 of the expression grammar |
EDC |
| Prevent Creation of First Instance for Repeating Forms Added by an Add Form or Add Event Rule | Auto-on | EDC |
| Status Change Date for Set Subject Status Rules | By Study Build | EDC |
| Select All for Assessments Item Group Visibility | Auto-on | EDC |
| Study Administration | ||
| User Activity Report | Auto-on | All |
| EDC Tools Functional Enhancements | Auto-on | EDC |
| Role Management & Security | ||
| Removed Manage Study Lock Permission from CDMS Deployment Administrator | Auto-on * In vaults where Role by Study is enabled |
All |
| New "View Coding" Permission | Auto-on * In vaults where Role by Study is enabled |
All |
| New "Workbench Tab" Permission in Role Management | Auto-on * In vaults that contain the Data Workbench application and where Role by Study is enabled |
All |
| Allow Deletion of Custom Study Roles | Auto-on * In vaults where Role by Study is enabled |
All |
| Manage Custom Object Permissions in Role Management | Configuration * In vaults where Role by Study is enabled |
All |
| Custom Object Support in Role Management | Auto-on * In vaults where Role by Study is enabled and qualifying custom objects are configured |
All |
| Download Study Role Permissions Report as an Excel™ File | Auto-on * In vaults where Role by Study is enabled |
All |
| Provide Federated ID during User Creation (UI) | Auto-on | All |
| Separate Security Profiles for Data Entry Study Roles | Auto-on * In vaults where Role by Study is enabled |
All |
| Deployments | ||
| Create a Production Environment from EDC Tools | Auto-on * In studies using the automated deployment model |
All |
| Integrations | ||
| Veeva Learning Training Report | Auto-on * In studies where the Veeva Learning integration is enabled |
All |
Enablement Legend
| Enablement | Description |
|---|---|
| Auto-on | Automatically activated and no configuration is required before using the feature; note that in some cases, a new feature is dependent on another feature that must be enabled or configured. |
| Study Feature Flag | This feature is available by configuration within the Study Configuration object (or similar). To enable a feature using study configuration, navigate to Admin > Business Admin > Study Configuration and edit the Study Configuration record for your Study. |
| By Study Build | The configuration options for this feature are available automatically in Studio, EDC Tools, Coder Tools, or System Tools, but you must configure them within your Study for those options to apply. |
| Support | On/off option controlled by Support. |