New Features in 25R2 Limited Releases
25R2 Planned General Availability: July 25 & August 1, 2025
25R1.4 Release Date: June 20, 2025
25R1.3 Release Date: May 30, 2025
25R1.2 Release Date: April 25, 2025
We are pleased to bring you new functionality with each limited release. These release notes are updated with upcoming new features one week before the limited release date.
Enablement Changes: The enablement of each feature is subject to change from release to release. For limited releases in the same general release, we will update this page. Enablement may also change before the general release. Refer to the Release Impact Assessment for the most up to date enablement for a general release.
Clinical Data
Features in this section are changes that apply to all application areas of Veeva EDC and CDB.
Include Deleted Data in PDFs 25R1.3
Use Case
To further clarify data revisions in the audit trail seen directly within the PDFs, the appendix now includes cases where data are removed.
Description
When generating closeout PDFs or running the Detail PDFs job, if a lead data manager chooses to include the audit trail, data that was removed by sites or a retrospective amendment will be included in the new Appendix section of the PDFs, “Deleted Data Audit Trail”. Bookmarks in the PDF follow the same order as the casebook schedule for easy navigation to the new section.
The following cases are included in the deleted data.
- Retrospective amendment with destructive design changes
- Site reset an event or form which was marked for removal
- Site reset a repeating event
- Site removed row in a repeating item group
- Site removed the form linking by deleting a linked form
- Query deletions, undo action in Quick Queries*
- Unscheduled Event is deleted
* The Undo action in Quick Queries is captured as a delete action. It’s included in the audit trail for the queried Item or Event. It’s only included in the Deleted Data Audit Trail section if the queried Item or Event is also deleted.
Restricted Data
Only users with access to restricted forms (users whose study role grants the Restricted Data Access permission) will see the Deleted Data Audit Trail. If the user who generated the file doesn’t have restricted data access, and there are deletions of restricted data, then Vault includes a note at the end of the PDF:
Note: The Deleted Data Audit Trail was not included in this file because the user who generated it did not have restricted data access. To include this data, please generate the file again as a user with restricted data access.
Enablement & Configuration
This feature is automatically enabled with the release.
Clinical Data Naming Update in PDF Export 25R1.2
Use Case
For clarity and simplicity, we have updated the name of the system to Veeva Clinical Data.
Description
This release updates PDF exports in the Clinical Data system to reflect the name change to Veeva EDC.
Enablement & Configuration
This feature is automatically enabled with the release.
Replace Non-Permitted Characters in PDF File & Folder Names with Hyphens 25R1.2
Use Case
The formatting of the file and folder names for the Detail and Closeout PDFs has been updated to further comply with industry recommendations.
Description
Resulting file and folder names previously using an underscore will now use hyphens (HYPHEN-MINUS, U+002D) to replace special characters found in the Study Name, Site Name, and Subject ID. When generating the Detail PDF, Closeout PDFs, and PDFs of individual forms or casebooks, any of the following non-permitted characters will be replaced with hyphens:
- Periods (.)
- Whitespaces
- Exclamation points (!)
- At signs (@)
- Pound signs (#)
- Dollar signs ($)
- Carets (^)
- Percent signs (%)
- Asterisks (*)
- Commas (,)
-
Pipes ( ) - Plus signs (+)
- Quotation marks (“)
- Tildes (~)
- Less than or greater than, angle brackets (< or >)
- Curly brackets ({ or })
- Forward or back slashes (/ or )
- Equals sign (=)
- Brackets ([ or ])
- Underscores (_)
In the folder and file names, uppercase characters are replaced with lowercase characters.
Notable Exception: When deploying a study, if the user chooses to include Detail PDFs in the deployment file, the file names will not include the replacement hyphens. These files are for internal record keeping and not for regulatory review.
Enablement & Configuration
Contact Veeva Support to enable this feature.
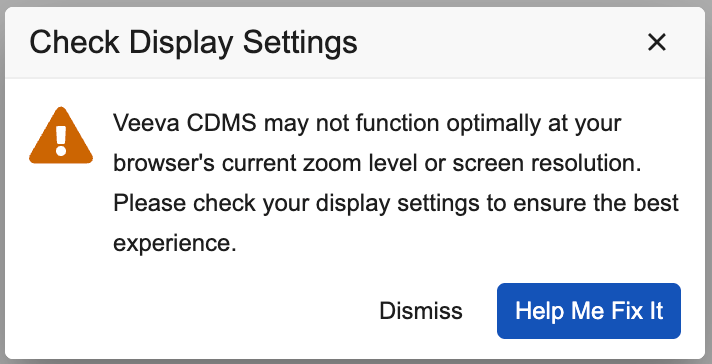
Screen Size Warning Adjustment 25R1.2
Use Case
This update reduces the occurrences of the screen size warning dialog displaying to end users to provide a better UI/UX experience.
Description
The threshold has been lowered for triggering display of the screen size and resolution warning dialog, which will now display to users for screen widths < 1270 or heights < 550. The warning is now displayed when the application becomes unusable rather than when it’s sized below the recommended screen size.
Enablement & Configuration
This update applies automatically.
Learn More
Data Entry
Features in this section are changes to the Data Entry tab, a working area for investigators and clinical research coordinators to enter study execution data.
Default Data Retained on ILB and Reset 25R1.3
Use Case
These changes make it easier to identify what default data was and was not collected.
Description
With this release, the following changes have been made to the treatment of default data when set as Intentionally Left Blank (ILB) or upon form reset:
- Default items are reset to the original defaults when the Form or section is reset, as they appeared when the Form was first created. This results in a lower burden on the site to understand if they need to add new rows to take advantage of added defaults.
- Row/section resets will retain values
- Form resets will rebuild the defaults with the correct number of rows and the correct defaults for that casebook version.
- When a row or Form is marked as ILB, the read-only default values are retained.
- Rows containing one or more default data items cannot be removed.
Enablement & Configuration
This update is automatically available for new Studies.
Edit Value Button Hidden for Answered Queries 25R1.2
Use Case
This update changes the query dialog to help site users easily identify queries that have already been answered.
Description
When a query is in an answered state, the Edit Value button will now be hidden. Note, the user will still be able to edit the value where the query is shown by clicking the Edit Form button. Similarly per the existing behavior, when the query is in an Open state, the Edit Value button will be enabled.
Enablement & Configuration
This update is automatically available.
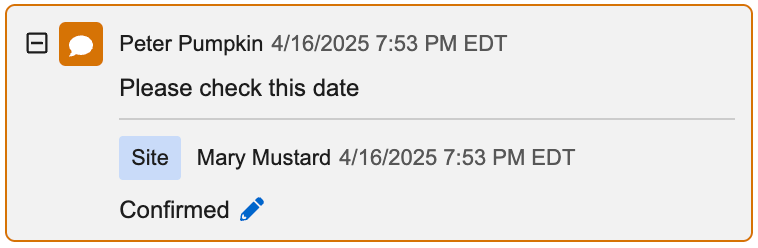
Quick Queries 25R1.3
Use Case
Sites and CRAs can eliminate the need to manually type out query messages and replies when using Quick Queries. Site users and monitors frequently use similar messages when opening and replying to queries, using text such as “Please confirm”, “Please verify”, etc. Sites will often reply with “The data is correct”, “Data updated” and “Marked as blank” or something similar. Quick Queries provides a clean, simple, and automated way for sites and CRAs to provide standard query messages and replies, drastically reducing the keystrokes required from users when creating queries, updating data, and answering queries.
Description
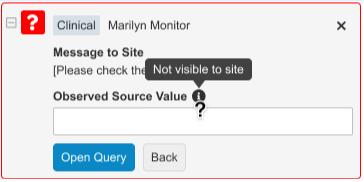
Standard query messages and responses provide a faster and better experience for Sites and CRAs. While opening manual queries, replying to manual queries, or replying to item property queries (edit checks) configured in Studio, the standardized options are made available based on the state of the data being entered or missing. Quick queries can be applied to data items and events.
CRAs are able to observe and record the source value within the query, which is easily referenced to compare and close the query after the site updates the data. Sites will not see the source value that the CRA included in the query, as it is only viewable within the Review tab and the audit trail if the user has the observed source value permission.
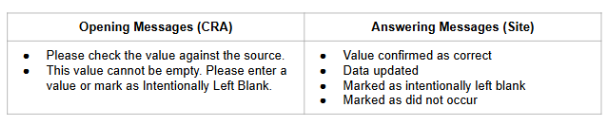
The available query messages and site responses will automate based on whether the item has a value or not. When opening a manual query, CRAs have three standard one-click options:
- Check Value Against Source (available when data is present)
- Enter Missing Value (available when data is missing)
- Create Custom Query
Site users will have the following one-click options to select for item queries:
- Edit Value
- Mark Intentionally Blank (when data is missing)
- Confirm Value is Correct (available when data is present)
- Reply with Comment
Similar options are available to site users for event queries:
- Enter Event Details
- Edit Event Details
- Mark as Did Not Occur (when data is missing)
- Confirm Value is Correct (available when data is present)
- Reply with Comment
The Create Custom Query and Reply with Comment options provide the CRAs and sites the option to manually type query messages and answers when needed.
When the site selects Edit Value, they must provide a reason for change. When this occurs, the system immediately puts the form to In Edit. The query will automatically be answered with the reply [Data updated] if the site user edits the value in the queried item or event.
Standard Query Messages for 25R2
System queries answered using Quick Queries will auto close when the conditions are no longer true, indicated with [Closed]. System-closed queries will not display on the form, but will be visible in the audit trail. Quick query messages are seen with brackets around the text [ ] both in the UI and within the extracts.
Permissions
Quick Queries adds two new permissions (Edit Observed Source Value, View Observed Source Value) to the following standard roles. These permissions are only effective when the study is using Quick Queries.
- Edit Observed Source Value
- CDMS Clinical Research Associate
- CDMS Super User
- View Observed Source Value
- CDMS Clinical Research Associate
- CDMS Super User
- CDMS Data Manager
- CDMS Lead Data Manager
Extracts & Listing Updates
The following new columns have been added to the Query Details Listing: Value Confirmed (Yes/No) and Observed Source Value. When using Quick Queries, the query message text will appear in the Original Query Text, Latest Query Comment, and Latest Query Answer Text columns.
In the SDE, we’ve added the Observed Source Value (OBSSOURCEVAL) column to the SYS_Q dataset and Quick Action Type columns (QUICKACT as Y/N and QUICKACTTYPE) to the SYS_QT dataset. The auto responses will appear in the system extracts as follows.
- SYS_Q and SYS_QT:
- QTEXT
- QTEXTBASE
- QTEXTENG
Enablement & Configuration
This feature is available in Studies where the Query Teams feature is also enabled. To use Quick Queries, studies also require Query Teams. Sites must be part of the Site Team and CRAs must be part of the Clinical Team. For existing studies, the Study Setting can be revised and deployed.
Learn More
Translations for “Subject Already Exists” Error 25R1.3
Use Case
The use of study language settings help convey to site users why a subject cannot be entered.
Description
With this feature, the system-provided error message seen in Data Entry for “Subject Already Exists” will be translated to the selected study language when the Study Language study setting is enabled for the study. If the Study Language setting is not enabled, then the message will default to the user’s language. This feature only applies when adding subjects using manual Subject ID generation. The English-language message is “The subject name “{subject ID}” already exists in the site.”
Enablement & Configuration
This update is automatically enabled.
Data Review
Features in this section are changes to the Review tab, a working area for clinical research associates and data managers, or to review functionality within the Data Entry tab.
Updates to Form Summary Fields 25R1.2
Use Case
This update ensures consistency across all cycle time calculations and supports reporting on cycle time metrics in Workbench and Clinical Reporting.
Description
The following Form Summary Cycle Time metrics will now return null values if the cycle time calculation is negative:
- Update Visit to Form Submit Cycle Time
- Update Submit to Signature Cycle Time
- Update Submit to SDV Cycle Time
- Update Submit to DMR Cycle Time
- Update Submit to Frozen Cycle Time
- Update Submit to Locked Cycle Time
In addition, the calculation for the Submit to Signature Cycle Time metric has been updated to use two decimal places.
Enablement & Configuration
This update is automatically available.
Clinical Coding
The following are new features for Veeva Coder, the clinical coding area for Veeva Coder.
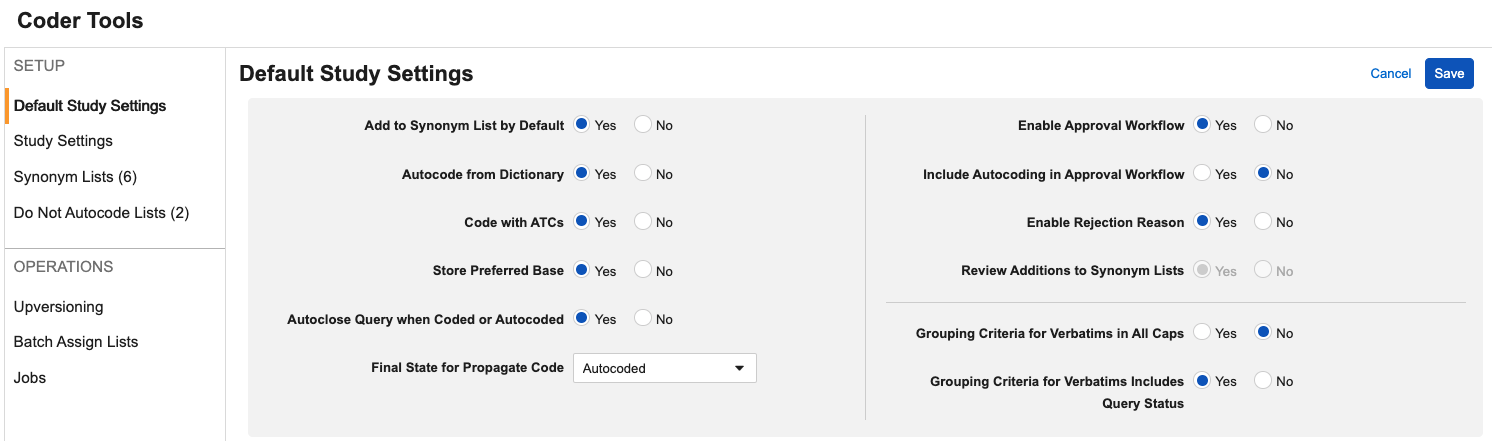
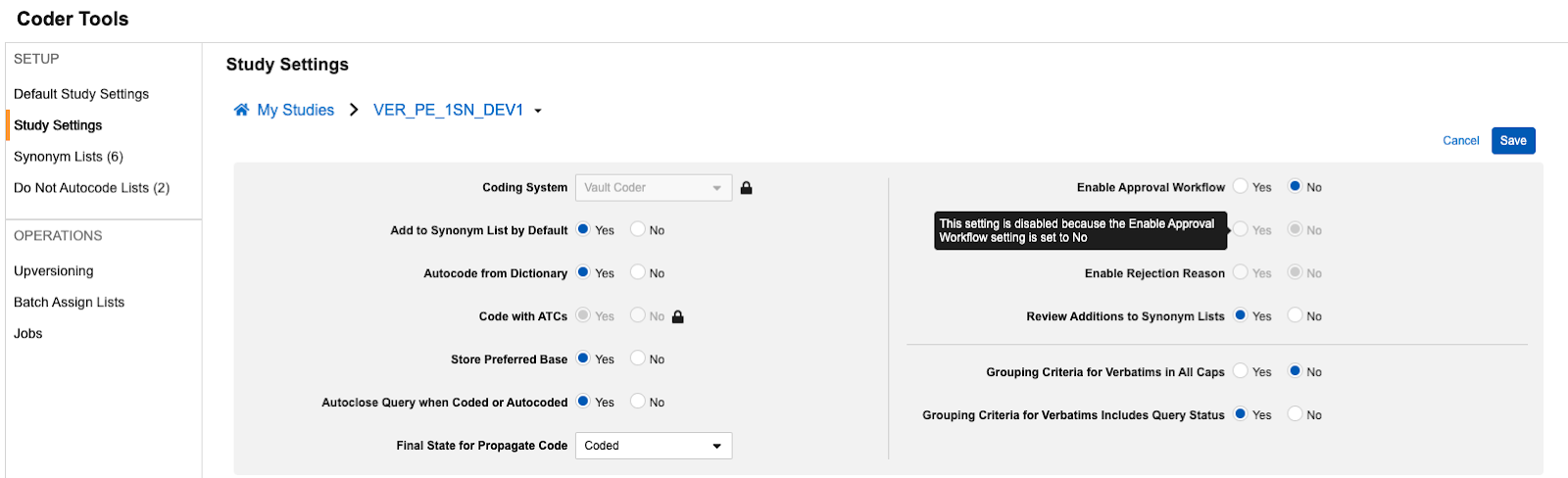
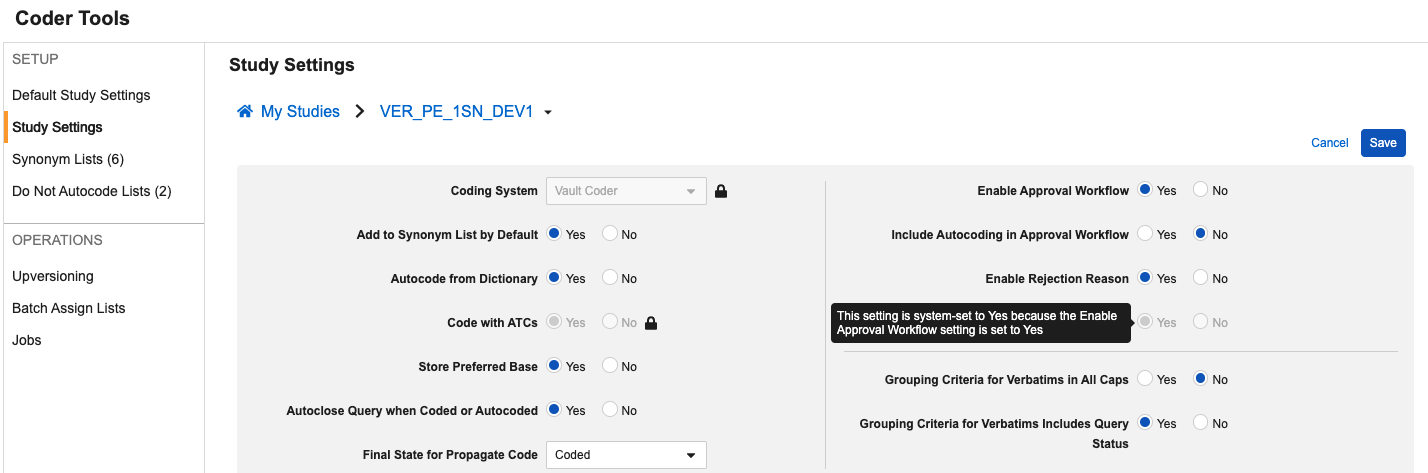
Option to Include Autocoding for Approval 25R1.2
Use Case
In order to support coding consistency and the accuracy of review in Veeva Coder, when the approval workflow is enabled for a Study, Coder Administrators can now configure Autocoded terms to also go through the approval workflow. This update removes the need to rely on reports or extracts to perform these reviews externally.
Description
This feature adds a new Coder Tools Study/Default Study setting to Include Autocoding in Approval Workflow, which is enabled only when the existing setting Enable Approval Workflow is set to Yes.
This setting can be implemented as a Default Study Setting or individual Study Setting. When set to Yes, any terms that are autocoded due to a Synonym List or exact dictionary match will move to the Pending Approval status, and upon approval will move to a final status of Autocoded. The default value for this setting is No.
To support this addition of this field to the UI and provide more clarity to user administrators, we’ve also reorganized the settings layout and added additional tooltips. Tooltips are visible when hovering over the options while in Edit mode.
Enablement & Configuration
These updates are automatically available after the release. Existing and new studies will have a default vault of “No” for the “Include Autocoding in Approval Workflow” setting.
Learn More
Sync Coder Lists Enhancements 25R1.3
Use Case
This update supports improved synonym list maintenance for customers managing studies across multiple production vaults using the EDC Sync Coding Lists Vault connection.
Description
Prior to this release, customers with multiple PROD vaults using the EDC Sync Coding Lists Vault connection could only use the options to Apply to Synonym List when approving or rejecting a code and the Propagate Code function in the primary Prod Vault. This release makes these functions available in the secondary Prod Vault, supporting bi-directional updates to the synonym list.
Additionally, the connection name has been updated from CDMS Sync Synonym List to EDC Sync Coding Lists, since the connection includes Do Not Autocode lists.
Enablement & Configuration
This feature is automatically available in vaults using the EDC Sync Coding Lists connection across multiple Production vaults.
Imaging
The following are new features for Veeva EDC Imaging, the imaging exam module for Veeva EDC.
Imaging: Support 1GB DICOM Files 25R1.3
Use Case
The supported file size threshold for DICOM exams has been expanded from 50 MG to 1 GB, which allows Imaging to accommodate larger files within an exam, improving upload assurance and capability.
Description
Site users can now upload larger sized DICOM files, up to a 1 GB file size for a single file in an exam. Updated notations in the UI remind site users as they are uploading files, Unzipped DICOM (up to 1 GB per file, 4 GB total).
Enablement & Configuration
This update is automatically available for studies using Veeva EDC Imaging.
Learn More
Imaging: Patient Name Assigned as SubjectID During De-Identification 25R1.2
Use Case
To provide traceability within downstream viewing applications, the metadata for Patient Name contained in uploaded DICOM files is now assigned the Subject ID value during the de-identification process.
Description
During the process of uploading DICOM file(s), the system automatically de-identifies specific PI/PII metadata including the Patient Name. Previously, the system assigned an anonymized value for the Patient Name as part of the de-identification process. With this release, the Subject ID is now assigned to this field. The Subject ID will be seen when the file is downloaded or viewed outside of EDC.
Note: Within EDC there is no visible change in the UI for Data Entry or Imaging end users. In EDC, the Patient Name will show the De-Identified tag:

This update applies only to the metadata value seen when viewing the file in downstream systems.
Enablement & Configuration
This update is automatically available for studies using Veeva EDC Imaging.
Learn More
UX: Updated Keyboard Operation in Data Grids 25R1.3
Use Case
Keyboard operations for Imaging data grids make navigation easier.
Description
When navigating Imaging data grids using arrow keys, the focus will move from cell to cell based on the key pressed, with the focus being placed on interactive elements within the cells. A blue box will display on actionable elements (e.g., links, action menus, sortable columns) and a grey box will surround unactionable cells.
Enablement & Configuration
This update is automatically available for studies using Veeva EDC Imaging.
Reports & Dashboards
The following are new features for reports and dashboards in Veeva Clinical Data.
Extracts - New Standard Reports 25R1.3
Use Case
These new standard reports provide improved visibility of information to Coder users, Lab Data Managers, and Vault Administrators as well as minimize the need to develop custom reports.
Description
This release introduces the following new and udpated standard reports:
- Standard Template: MedDRA Coding Report (V4) - V4 updates the reporttype to include Coder notes.
- Standard Template: WHODrug Coding Report (V4) - V4 updates the reporttype to include Coder notes.
- Standard Template: JDrug Coding Report (V4) - V4 updates the reporttype to include Coder notes.
- Standard Template: Lab Locations and Sites - This new standard report includes lab locations and the sites using those locations.
- Standard Template: Lab Reference Ranges - This new standard report lists lab locations and the normal ranges they use.
- Standard Template: Lab Item and Analyte Definitions - This new standard report details lab item definitions and analyte definitions used within a study.
- Standard Template: Study Masters and License Keys in EDC Tools - A new standard report for Vault Owners to view the license key entered for each study in the Vault. (Note - the data in this report will only be visible to Vault Owners)
Enablement & Configuration
These reports are immediately visible to Vault Owners. Vault Owners must share the new Coding and Lab reports in order for them to be visible to other users.
Learn More
Assessments
The following are new features for the Assessments area of Veeva EDC.
Subject Filter Added to Assessments Grid 25R1.2
Use Case
We’ve added a new Subject filter to make locating and searching for subjects within the Assessments tab easier.
Description
The Assessment grid now includes a Subjects filter, which is located between the existing Site Number and Reassessments filters. The new text search is not case sensitive, alphanumeric, and will search subject IDs from left to right.
Enablement & Configuration
This update is automatically available.
Study Design & Configuration
Features in this area apply to Studio, the study design and configuration area for Veeva EDC.
Schedule Grid Improvements for Dynamic Rules 25R1.2
Use Case
These enhancements for viewing dynamic rules provide better usability and clarity for study designers when working within the Studio Schedule Grid.
Description
Updates seen within the Studio Schedule Grid include:
- A new icon for dynamic Forms, Events, and Event Groups where no rule is configured. On hover over the icon, the tooltip message displays “No rule. Click to add.” and opens directly to the Rule Editor on click.
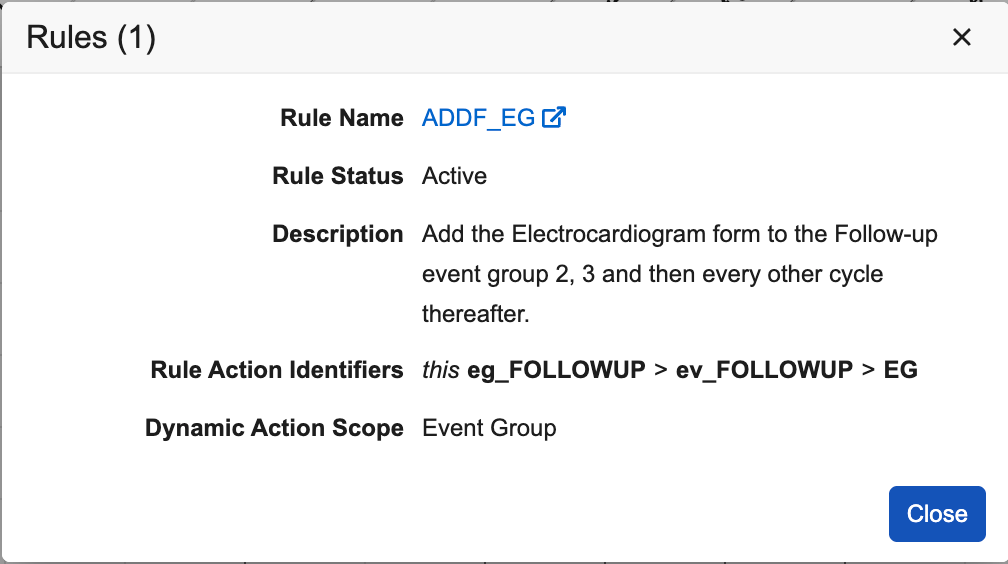
- Count of rules (active + inactive) configured for the dynamic object: “View rules (#)” appears when hovering over the dynamic rule icon ().
- New dialogue when clicking the dynamic rule icon displays additional details of the rule(s).
Enablement & Configuration
These updates are automatically available in Studio.
Learn More
Local Labs: Fasting Status & Female Cycle Support for Normal Ranges 25R1.3
Use Case
Lab Normal Ranges can now be managed based on Fasting Status and Female Cycle, eliminating the need to maintain duplicate analytes for these scenarios.
Description
Lab normal ranges can now use Fasting Status and Female Cycle as parameters in the same way that Sex, Age Range, and Effective Date are currently used. Lab forms can be configured to collect Fasting Status and/or Female Cycle at the time of collection where applicable. This information can then be used to identify the appropriate normal range value.
Studio
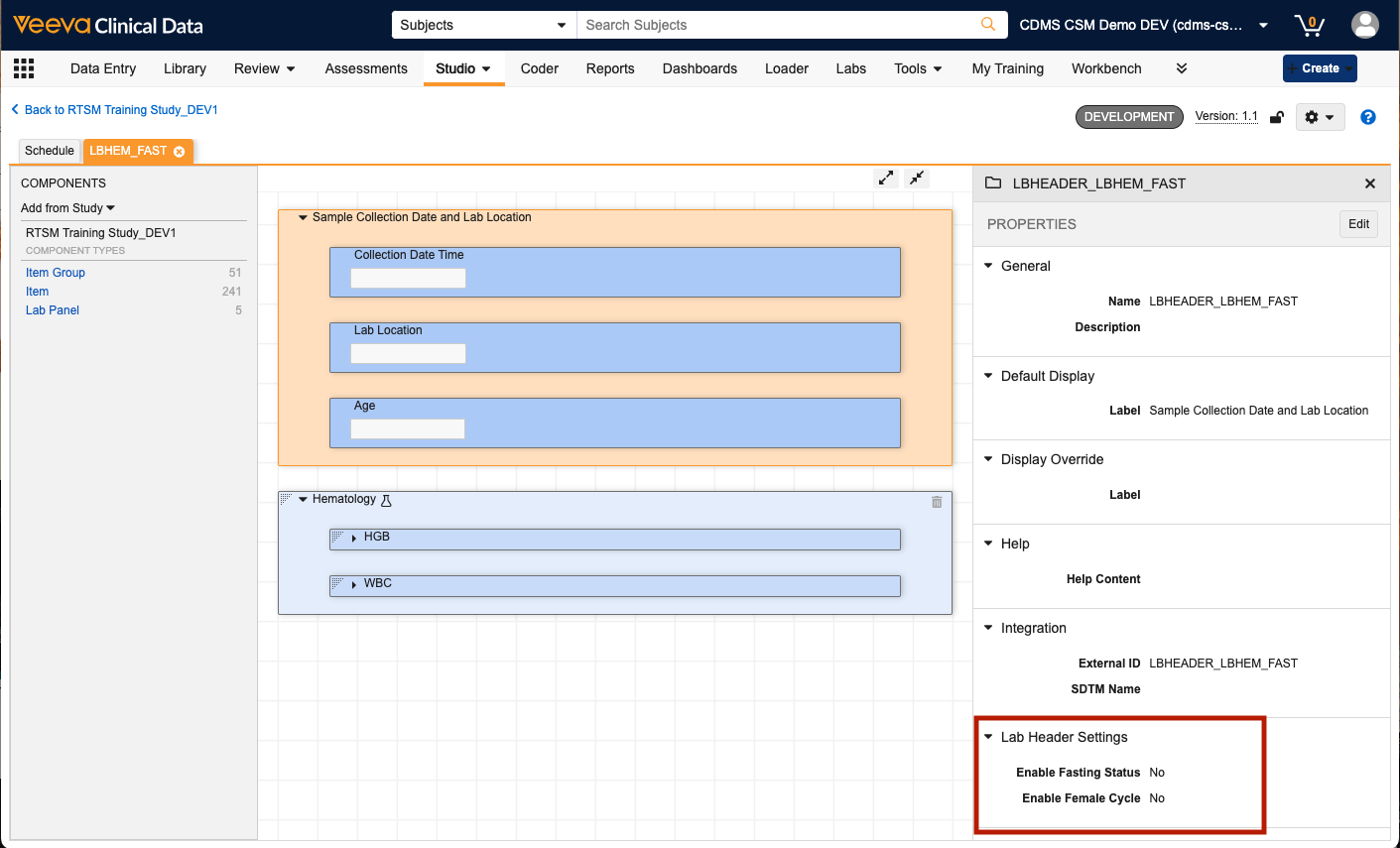
Study builders can indicate if a lab form should include Fasting Status or Female Cycle in the lab header by modifying the Lab Header Settings in the Item Group property panel:
Selecting Yes triggers the addition of system-generated LBFAST and LBFEMALECYCLE items to the Lab Header for the form. These items will also be included in Extracts, Clinical Reporting, and CDB, if applicable.
To support this change, we have also updated the naming convention for the Lab Header item group. Previously, all Lab Header item groups were named LBHEADER and were reused across all lab forms as the items within the item group were always the same. With the new option to selectively add Fasting Status and Female Cycle to certain lab forms, the Lab Header item group is now unique for each lab form. As a result, the new unique item group name will be LBHEADER__FormName_.
Lab Settings
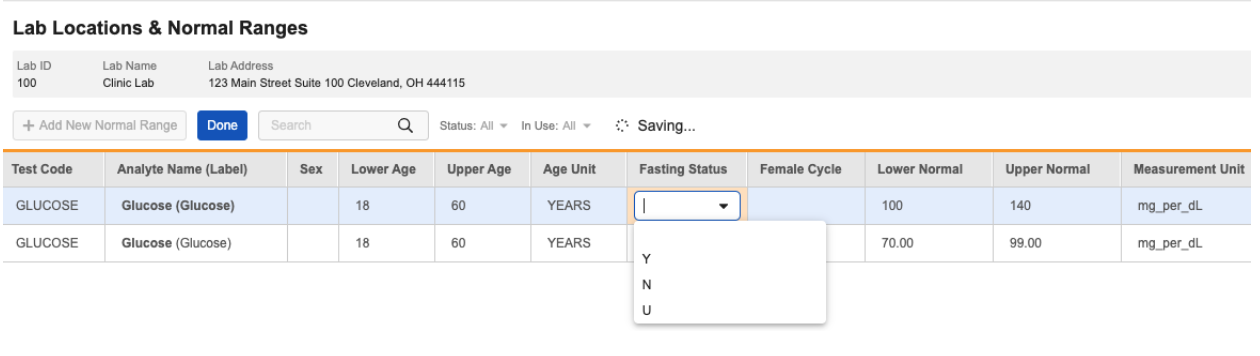
We’ve added a new Labs codelist for Fasting Status to System General Settings, with options Yes, No, and Unknown. The Female Cycle codelist was added in a previous release. Vaults created after 25R1 include an option for Pregnancy in this codelist. Users can add this option in older vaults if needed.
Normal Ranges
The Normal Ranges grid has been updated to include columns for Fasting Status and Female Cycle by default. These columns can be moved or removed using the Edit Columns option.
Lab Data Managers can configure normal ranges for analytes impacted by Fasting Status or Female Cycle as needed.
The Normal Ranges import file has also been updated to include an optional column for Fasting Status. The Female Cycle column was added in a previous release.
When evaluating normal ranges, the system will first try to match an exact value for Female Cycle and/or Fasting Status (as applicable). If there is no exact match, the system will use otherwise matching ranges where Fasting Status or Female Cycle is blank. Therefore, if an analyte is not impacted by fasting status or female cycle, these values can be left blank in the normal range.
Data Entry
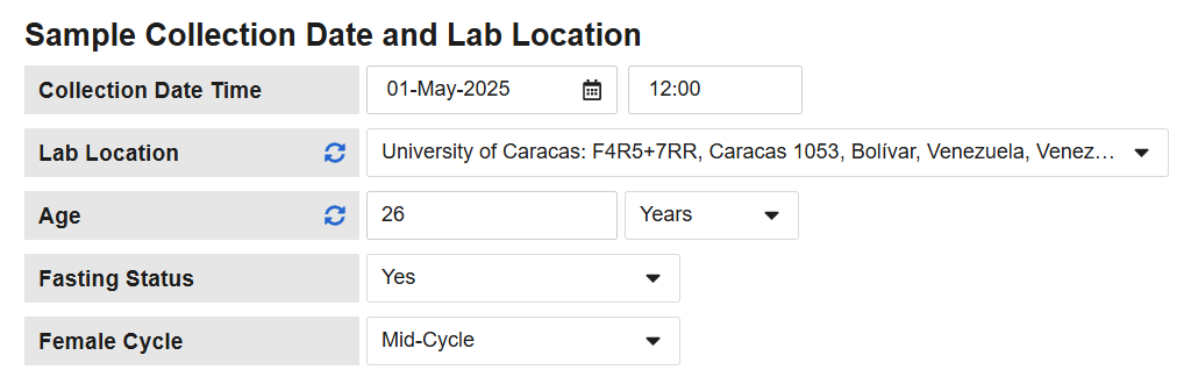
When a lab form is enabled to use Fasting Status and/or Female Cycle, the form header will display the related questions to the Site. Data Entry users must provide a response or mark the item as Intentionally Left Blank in order to enable the lab panel and load normal ranges.
Enablement & Configuration
This feature is available in new studies. It can be added to existing studies with the creation of new lab forms.
Learn More
- Configuring Labs in Studio
- Managing Lab Locations, Normal Ranges, & Reports Mass Updates
- Study Data Extracts
Local Labs: Study Settings Error Messaging UI Update 25R1.3
Use Case
This update ensures accessibility and improves the consistency and clarity of the user experience.
Description
All errors shown on the Labs Study Setting page due to an invalid configuration have been updated to include a new error icon.
Enablement & Configuration
Auto-on.
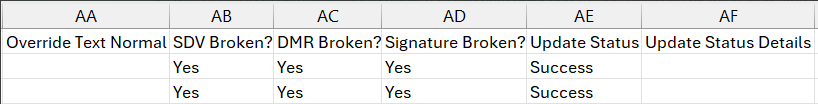
Local Labs: Update to Preview and Update Outdated Lab Normals Jobs 25R1.3
Use Case
Running the Outdated Lab Normals job can break Signature, SDV, and DMR if changes result from the job. This feature documents that outcome in the Preview and Update Outdated Lab Normals job output files.
Description
The following columns have been added to the Preview and Update Outdated Lab Normals job output files, all of which will have a Yes/No value:
- SDV Broken?
- DMR Broken?
- Signature Broken?
When these columns are marked as “Yes”, review states will only be broken if the update is successful.
Enablement & Configuration
Auto-on.
Learn More
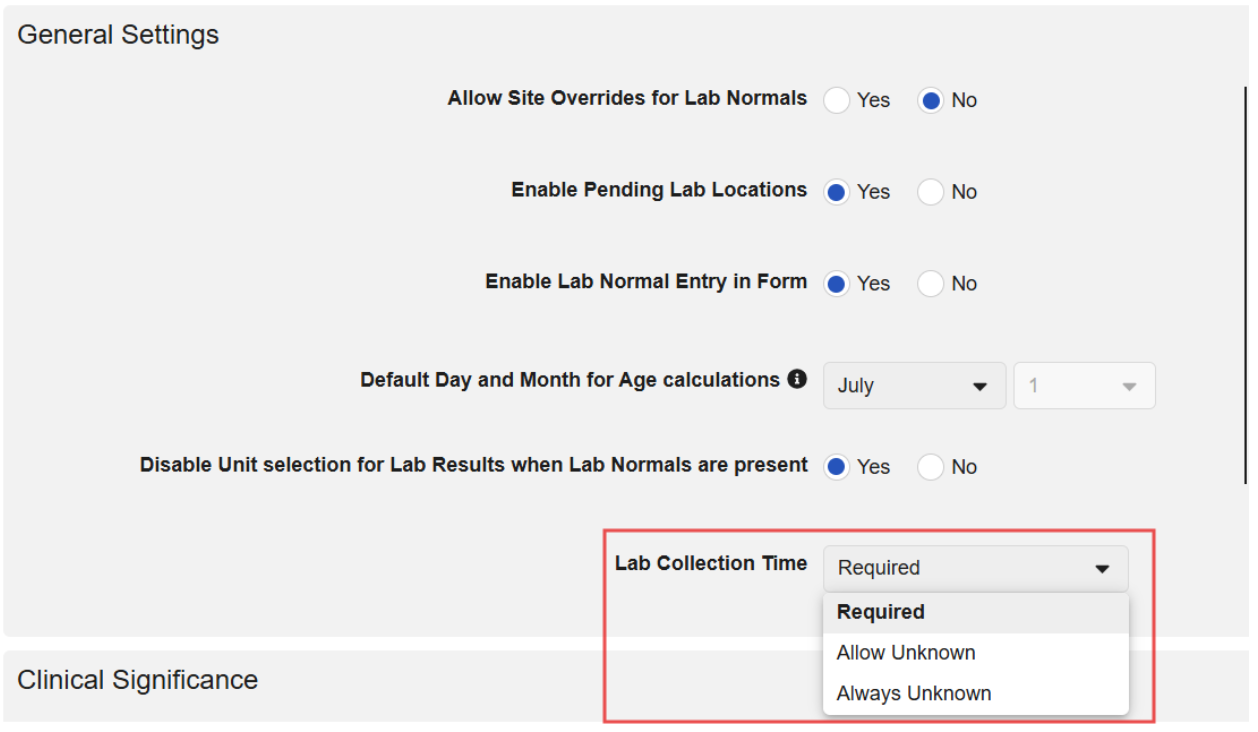
Local Labs: Time Unknown for Lab Collection Datetime & Normal Range Effective Datetimes 25R1.3
Use Case
Lab sample collection date and time is used for the evaluation of lab normal ranges. Prior to this release, collection time was required. Now, studies can be configured to support unknown time values, simplifying the site user experience when collection time is not provided by the lab. Additionally, we have added the ability to enter unknown time for normal range effective datetimes for scenarios where the time is not provided by the lab.
Description
Lab Settings
Sponsors can now choose if lab collection time is required, allows unknown values, or is always unknown. This setting can be implemented from within System General Settings (default settings for new studies), and can also be set at the study level in Study General Settings.
The selected setting value is included in the Study Design Specification (SDS) exported from Studio.
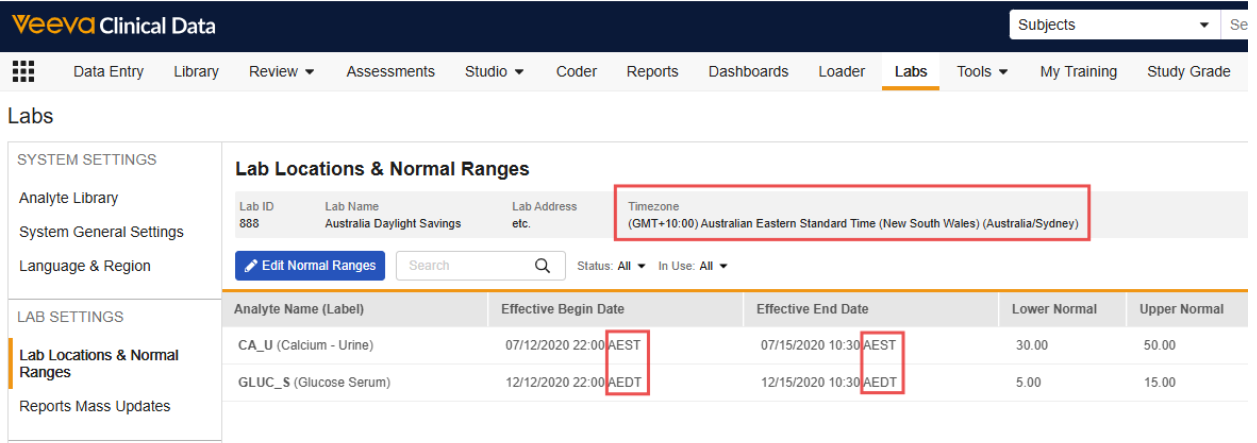
Lab Location: Timezone Collection
Vault will now collect Timezone for each lab location to support more precise evaluation of Effective Start and End Dates/Datetimes for lab normal ranges and easier entry for Lab Data Managers. This field is required for newly created lab locations after the release, and is displayed in the Lab Locations grid and in the header of the Normal Ranges grid. All normal range effective datetimes entered for a lab location will be in the Timezone of the lab location.
For lab locations existing at the time of the release, the Timezone field will be populated in the following order as applicable:
- Based on the entered address and country value for the lab location, if possible
- Based on the timezone of the site to which the lab location is related, if the first option isn’t possible
-
If the lab location is not associated with any sites, the lab location timezone will be updated to match the Vault timezone
- Customers are strongly encouraged to review the timezone settings for their lab locations after the release and edit as needed.
Lab Location Import File Updates
Lab Locations can now be updated via import to support better management of Lab Locations, including updating timezone values where needed after the release. Previously, the import supported only the creation of new lab locations.
- When updating lab locations, the LabID is required. Other field values can be provided if the values are being updated. Blank values will not override existing values.
- When creating or updating lab locations via import, supported timezone values should be used.
Lab Normal Range Management and Evaluation
The Effective Start and End Datetime values now support Unknown time. Lab Data Managers can use the question mark ( ? ) option when the time is unknown. With this change, time values must be entered in a 24-HR format.
All entered datetime values will be evaluated based on the timezone of the Lab Location. This will be compared to the Lab Collection Datetime value entered by the site which is evaluated based on the Site timezone.
Extracts
In the Study Data Extract (SDE) and EDC Core Listing, unknown time values will be output as UN:UN:UN.UNKZ and the data type of the field will be a string / char (SAS datasets). The length will be updated to 64 (SAS length 256).
If CDB is used, the datetime data type will be preserved, and the input time of 12:00 will be used. The datetime field’s RAW value will be displayed as a string using UN:UN.
Post Go Live Restrictions
A new casebook version is required to update the Lab Collection Time setting after deployment of a study to Production.
Enablement & Configuration
This feature is immediately available. Studies existing at the time of release will be set to require full date and time.
Learn More
- Managing Analytes, Units, & Codelists
- Managing Lab Locations, Normal Ranges, & Reports Mass Updates
- Study General Settings for Labs
- Entering Lab Results
- Study Data Extracts
Repeating Item Group Data in Email Notifications 25R1.2
Use Case
Additional configuration options for email notification rules provide better visibility into data for email recipients.
Description
Email notification rules now support data coming from repeating item groups. When configuring a rule, study designers can add tokens for items located within repeating item groups to the Message area of the Send Email rule action. New tool tips for the Rule Action help guide study designers when including data from repeating item groups.
Email recipients will see the applicable data within one email notification for all item group repeats where the rule logic evaluates to true.
Enablement & Configuration
This update is automatically available in Studio.
Learn More
Rule Editor Supports Country Code on @StudyCountry 25R1.2
Use Case
This feature allows study designers to program rules based on Veeva Vault Platform’s standardized country code values. The standard country codes, added as part of the 24R3 general release, provide a seamless connection between countries across different applications regardless of differing country names. These codes can now be used in the Rule Editor to ensure the rule always references the correct study country, even if the country name is changed by the Clinical Operations - EDC connection.
Description
The country code, @StudyCountry.country_code__v, is now available when creating rule identifiers in the Studio rule editor. As a best practice, study designers should use this reference instead of the country name text string when configuring User Defined Rules.
Enablement & Configuration
This update is automatically available in Studio. For Vaults created prior to 24R3, please contact Veeva Support to ensure the latest country codes are updated for the Vault.
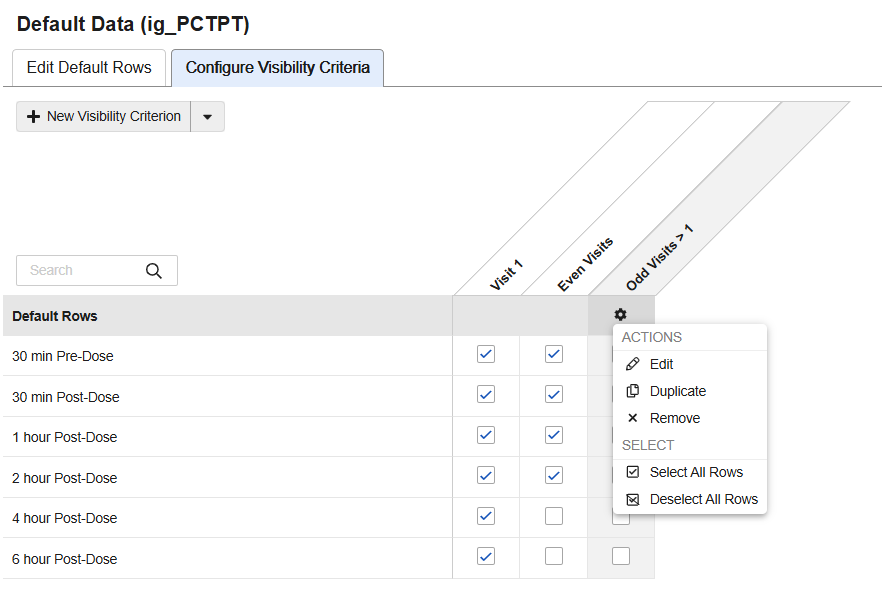
Schedule-driven Default Data 25R1.3
Use Case
New Studio design selections increase standardization and design reuse by allowing repeating item groups to be configured with different default data at specific Events, Event Groups, and Forms within the casebook. Study designers can reduce development and testing effort by decreasing the overall number of unique Forms and further reuse Item Groups across unique Forms as required for the study design. This also reduces downstream effort in programming to combine Form data in reports and extracts.
Description
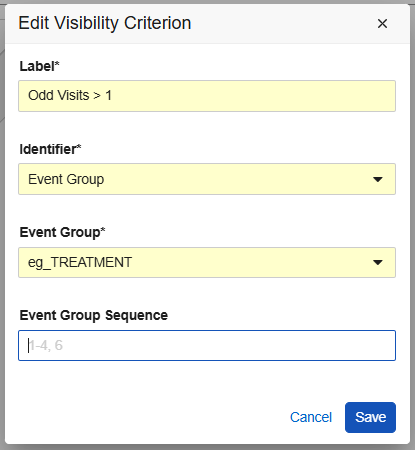
In the new Visibility Criteria tab, seen in the Default Data area of the repeating Item Group properties, study designers can set up the criteria that determines which default codelist item values are visible at various locations within the study schedule. After creating new visibility criteria, menu actions and checkboxes make it easy to see and select the rows to default. From the selection grid, study designers can select, hide or reorder the data defaults as required by the study protocol.
The default data can be assigned to specific Forms, Event Groups and Events. For repeating Event Groups and repeating Forms, sequence numbers can be entered to designate specific study schedule locations where the selected values will be defaulted. Configuration options dynamically adjust based on the identifiers and the study design. Validations and warnings assist designers to configure the default data correctly.
A new tab, Item Group Default Values, has been added to the Study Design Specifications (SDS) detailing the visibility criteria design configurations. Notations within the Annotated PDFs and appendix also include the visibility criteria. Following a deployment, changes to the criteria can be seen when running Comparison Reports in Studio. Visibility Criteria can also be copied from Studies and libraries.
Enablement & Configuration
This update is automatically available.
Learn More
Studio Copy Improvements 25R1.3
Use Case
This feature allows Study Designers additional capabilities when copying from libraries or other studies to further study design reuse and reduce efforts when configuring studies.
Description
Copying capabilities within Studio have been added and improved to include the following features:
- Copy Lab forms
- When the destination study has labs enabled, lab forms from the source study can be copied
- System rules are always included when copying forms
- Univariates and Progressive Display rules will always be included when copying forms, these are copied as part of the form properties and independent from the checkbox to include rules
- Added filters and improved searching when copying
- A new “Search by” filter in the copy dialog allows users to filter by All Fields, Name, Description, or Form
- When selecting the checkbox to include rules during a form copy, user defined rules evaluate against the schedule to further ensure rules are copied successfully, resulting in fewer skipped rules.
- Form copies will always run as a job, and provide a log regardless of the number of forms copied. This replaces the previous progress dialog when 20 or fewer forms were selected.
Enablement & Configuration
Automatically available in Studio.
EDC: Study Language & Locale for Blank PDFs 25R1.3
Use Case
This enhancement expands on the Study Language feature released in 25R1 to include Blank PDFs generated from EDC.
Description
When exporting Blank PDFs from Data Entry or Review, the PDFs will display in the specified study language and locale when the Enforce Study Language setting is set to “Yes”. If the study language is not enforced, then the Blank PDFs will be generated with the user’s language and locale.
Enablement & Configuration
Automatically available in Studio.
Study Language for Studio Labels 25R1.3
Use Case
Previously, the Enforce Study Language setting needed to be set to “Yes” in order to see and save translations in Studio. This feature allows study designers to see the translated labels in Studio to ensure that the build is in the study language, particularly when the study language is different from the vault and user languages.
Description
With this feature, the system uses the value entered for Study Language, independent from the Enforce Study Language setting, in order to show and save translations in Studio. The design object labels in Studio will be saved in the selected Study Language and where translations have been uploaded for the study. This includes labels for Event Groups, Events, Forms, Item Groups, Items, and Codelist value labels, etc. As part of this feature, Studio-generated PDFs will display in the selected Study Language. If a study language is not selected, the labels will default to the vault language.
Note that application labels (non-study labels) will still show in the user’s language.
Enablement & Configuration
Automatically available in Studio.
Study Locking & Billing Enhancements 25R1.3
Use Case
These updates improve billing status clarity for non-ELA customers.
Description
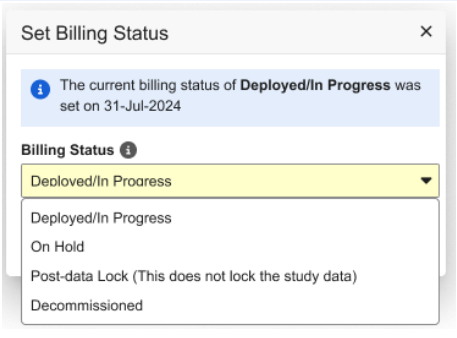
We have updated the Billing Status option labels for non-ELA (Enterprise License Agreement) studies to provide greater clarity.
- The Locked option has been renamed to Post-data Lock (this does not lock the study data). With this change, users will no longer be able to set the billing status to locked if the study data isn’t already locked.
- The Archived option has been replaced with Decommissioned. Casebook creation and study deployments are not allowed for studies in the Decommissioned billing status as with the previous Archived status.
These updates are also reflected in the related dialog boxes.
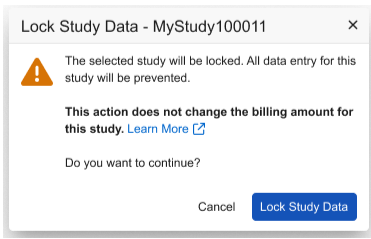
We’ve also updated the dialog box displayed when study data is locked, to clarify that study data lock does not affect the billing status of the study.
Enablement & Configuration
These updates are immediately available for non-ELA (Enterprise License Agreement) studies.
Learn More
Study Administration
Features in this section apply to System Tools or EDC Tools, a study-level administration area for Veeva EDC.
Admin: Support VeevaID Username Update 25R1.3
Use Case
In 25R2, Platform updates to VeevaID now allow a user to update their username (i.e., email address). This feature supports visibility of these changes within EDC.
Description
To support the 25R2 Platform feature allowing VeevaID users to update their email address, we are making the following changes in EDC to show this change:
- The User Activity Report has been updated to include username and email changes for both VeevaID and sponsor users.
- The User Access report now references both the new and old usernames when access events occur after a VeevaID user updates their email address/username.
Enablement & Configuration
These updates are immediately available.
Learn More
Enhancements to VeevaID & Veeva Training Reporting 25R1.3
Use Case
Users with multiple Veeva accounts may use a different account for CDMS Vault Training than for an EDC Vault. These updates clarify which username to use for training access.
Description
We are implementing the following enhancements to the Training Report and My Training tab:
- Two column changes have been made to the User Training Report. These columns update approximately every 12 hours:
- Learning System Username: The logic for populating this column has been updated.
- The report will display the VeevaID (site users) or Domain username (sponsor users) associated with the user’s training access.
- If the training username is not yet available and the CDMS user is “Sponsor,” the Learning System Username column will be blank.
- If the training username is not yet available and the CDMS user is “Site,” the Learning System Username column will display the CDMS user’s email address.
- If the CDMS user has no user type, the Learning System Username column will be blank.
- VeevaID Registration Status: This column has been added to the report and displays the registration status of the VeevaID user (Registered, Invitation Sent, Invite Attempts Exhausted, etc.).
- Learning System Username: The logic for populating this column has been updated.
- The Table on the My Training tab has been updated to display the training username for every training requirement.
-
When a user clicks the Training Access link on the My Training tab and needs a different username for login, the system will pre-populate the login screen with the correct Training username.
-
When the user in CDMS is the same as the user in Vault Training, the redirect to Vault Training has been improved to prevent re-authentication.
Enablement & Configuration
This feature is immediately available.
Learn More
Enhanced Tooltip for Audit Trail Export Jobs 25R1.2
Use Case
Tooltip enhancements provide added details to help end users quickly identify which job options were included in the audit job.
Description
Hovering over the information icon next to the Audit Trail Export job within Job History will display the details of the job. The selected Date ranges, Users, Sites, Subjects, and options for Include the Definition, Relationship and Facade Audit Changes; and Include Medical Coding Audit Trail will be seen within the tooltip. When no selections are made, then the information icon will be hidden. The selections can be seen in the following jobs:
- Audit Trail Export by Subject
- Audit Trail Export By Site
- Audit Trail Export by Study
Enablement & Configuration
This update is automatically available.
Rules Job: Close Queries for Inactive Rules 25R1.3
Use Case
Users with the Run Rules permission are now able to close queries that were opened by rules that are no longer active, reducing efforts for CRAs and Data Managers.
Description
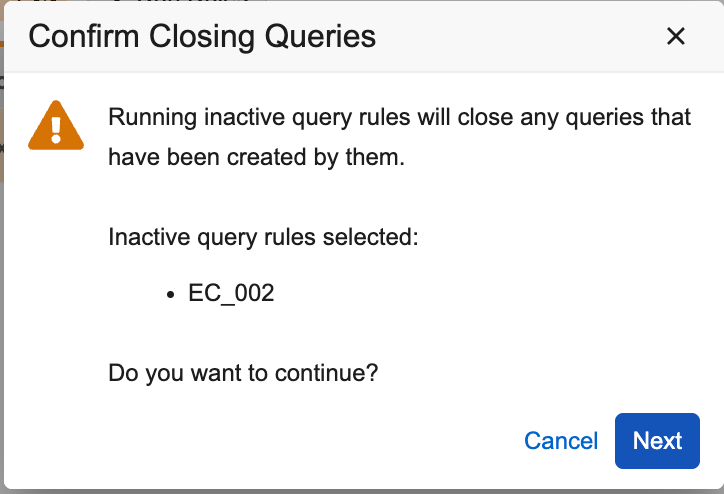
From the Run Rules job in EDC Tools, inactivated Open Query rules can be selected and run in order to close open queries that were created when the rules were still active. The Rule Status filter can be used to locate specific rules that were initially active and subsequently inactivated in Studio and then deployed. When running the inactivated rules, a confirmation dialog allows users to review their selections before running the job.
The output files from the rule preview job and the rule job will show the “Closed” Action in the associated job files. The Audit Trail and Query Details listings will show that the query was closed by System. Only queries in the Open status will be closed. Answered queries will not be closed. As part of this feature, the default view for Rules listed on the Rule job page is filtered to include only Active rules. Inactive rules can be added to the filter view by users when they need to run them. This helps prevent excess inactive query rules from being selected and run inadvertently.
Enablement & Configuration
This feature is automatically enabled.
Learn More
Do Not Display Origin Site for CTMS Entry in Audit Trail 25R1.2
Use Case
This update improves the readability of audit trails by no longer displaying unhelpful ID values that provide no meaning to the user.
Description
In 25R1, we released updates to the Move Subject function, including stamping the origin site on Events and Protocol Deviations to support site payments handled in Clinical Operations Vaults. In 25R2, we will no longer display the entry for the Origin Site for CTMS value in the Event or Protocol Deviation audit trail UI, Detail PDF, or Audit Trail Export output, as the displayed ID value provides no value to the end user. The removed audit trail entry was in the following format:
"Origin Site for CTMS" set to "[origin site ID number]"
The audit trail entry for the action of moving the subject to a new site remains in the audit trail.
Enablement & Configuration
This update is automatically available.
Imaging Extract Job 25R1.4
Use Case
Bulk extraction capabilities provide a more efficient way to download imaging exams, providing sponsors an easier way to receive and transfer exams to their storage locations for long term retention. This feature is especially beneficial for larger sites and studies that include large quantities of exam files.
Description
Imaging exams can now be extracted in bulk from a new, ad-hoc job available in EDC Tools, Imaging Extract. Lead data managers and admins can add a job that will transfer exam copies to a parent folder, imaging_extract, within the user’s Vault File Staging area. Options for including restricted data and to run selected sites or subjects are available when running the job. The option for restricted data is only visible if the user has the “Restricted Data Access” permission.
Upon successful transfer, users receive an email notification and can access the staged files via GUI clients or APIs to integrate with downstream systems. Users with the new “Vault File Manager Access” permission will be able to access the extracted images from File Staging using Veeva’s Vault File Manager client. Once the job completes, the files will be automatically removed from the file staging after 72 hours. The root folder will appear using the below format and contains subfolders for sites, subjects, and exams.
Study_Imaging_Extract_2024_05_27_11_25
Study_Imaging_Extract_2024_05_27_11_25_RESTRICTED
Please note that only one Imaging Extract job for the study may run at a time.
Enablement & Configuration
Contact Veeva Services to enable this feature. This feature is automatically enabled in the 25R2 General Release.
Learn More
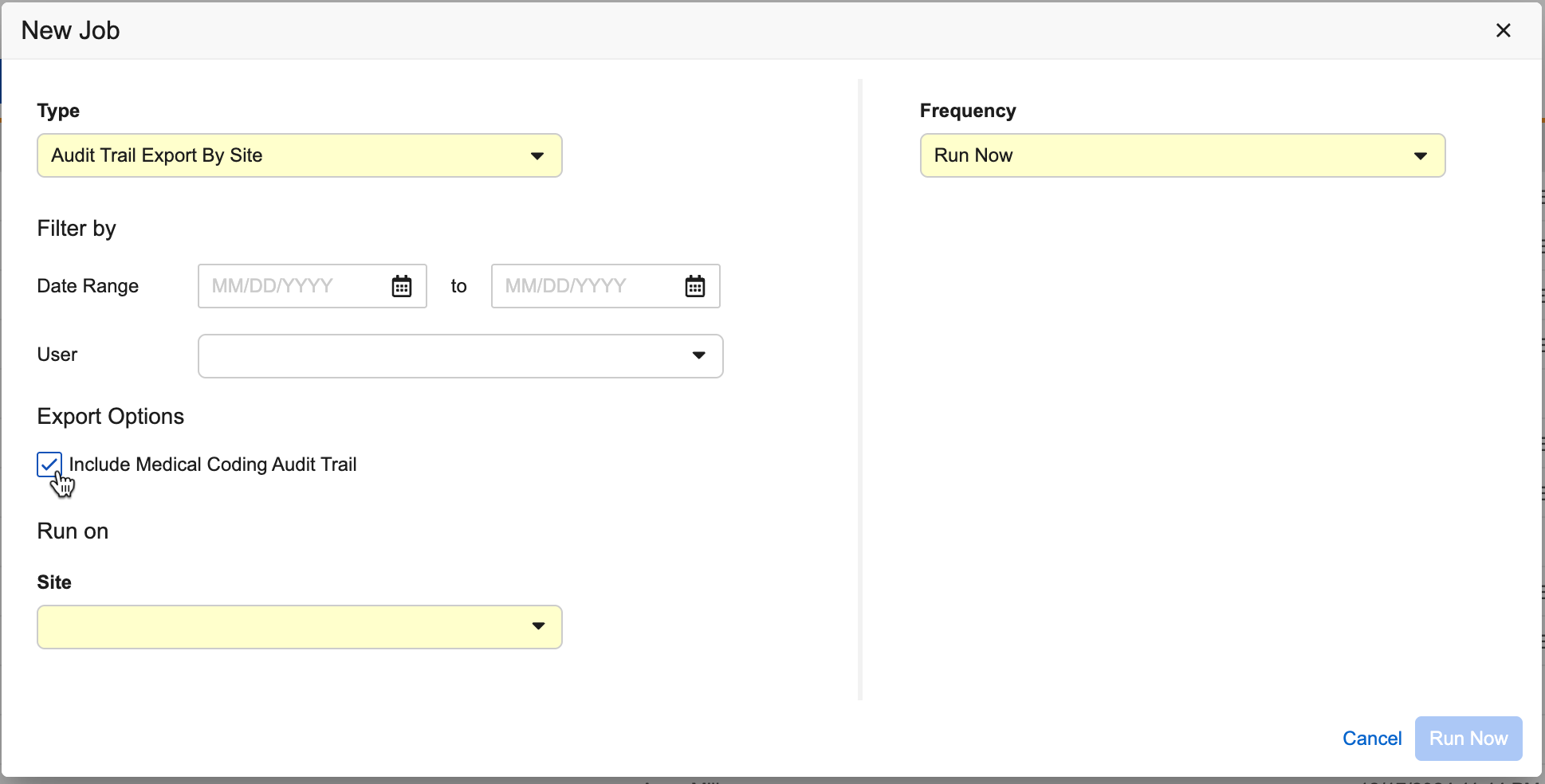
Include Coder Audit Trails in Audit Trail Export 25R1.3
Use Case
The Medical Coding Audit history was previously visible only from within the UI. This release now makes this information exportable as part of an Audit Trail Export for review and analysis.
Description
The Subject, Site, and Study Audit Trail Export jobs now provide the option to include the Medical Coding Audit Trail.
To support usability of these audit trails, we are suppressing references to Label Keys and ‘All Caps’ values from both the Audit Trail UI and Export. This includes the following fields:
- AE Action Taken Label Key
- AE Expected Outcome Label Key
- AE Outcome Label Key
- AE Relationship Label Key
- AE Seriousness Label Key
- AE Severity Label Key
- AE Action Taken AllCaps
- AE Expected Outcome AllCaps
- AE Outcome AllCaps
- AE Relationship AllCaps
- AE Seriousness AllCaps
- AE Severity AllCaps
- CM Course Number Label Key
- CM Dose Label Key
- CM Dose Unit Label Key
- CM Frequency Label Key
- CM Indication Label Key
- CM Route Label Key
- CM Course Number AllCaps
- CM Dose AllCaps
- CM Dose Unit AllCaps
- CM Frequency AllCaps
- CM Indication AllCaps
- CM Route AllCaps
- Verbatim AllCaps
Enablement & Configuration
This feature is automatically enabled.
Learn More
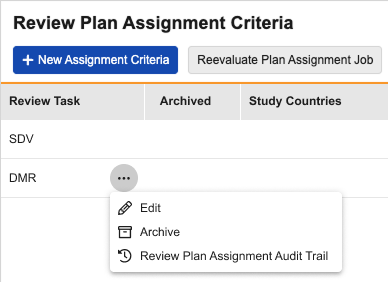
Review Plan Assignment Audit Trail 25R1.3
Use Case
Data Managers and users with the Manage Review Plan Assignment permission can now see the audit trail for review plan assignments within EDC. This feature provides customers with better oversight on review plan changes without having to contact Veeva Support.
Description
Review Plan Assignment Audit Trail has been added as an option to the action menu on the Review Plan Assignment Criteria page. Selecting the Review Plan Assignment Audit Trail option will open the audit trail dialog for the review plan assignment record, showing the Event Description, Username, and Timestamp in the timezone of the user viewing the audit trail.
As part of this feature, the Archived column has been added to the grid and to both the CSV and Excel exports. A new Show Archived Assignments checkbox allows users to filter their view. The checkbox is unchecked by default.
Archived review plan assignments cannot be un-archived. Users must add a new assignment record after archiving. A dialog will warn users when archiving a review plan with the following message:
- Review plans will not be assigned based on this criteria moving forward
- Existing review plan assignments are not impacted
- This action cannot be undone
- You must create a new record in order to use the same criteria again
Enablement & Configuration
Auto-on.
Learn More
S3 Extracts File Delivery Support 25R1.3
Use Case
Delivery to a customer’s Amazon S3 bucket expands options for delivering exports from Veeva EDC.
Description
A new external delivery option is available with this release: the ability to configure customer-owned Amazon S3 buckets to receive files from Veeva EDC and Veeva Coder. This additional delivery option allows jobs that are scheduled to deliver their files to the Amazon cloud after configuration in System Tools.
Enablement & Configuration
This feature is available to users with access to All Studies, System Tools, and the renamed permission, Manage Outbound Connections (formerly Manage FTP).
Clarify Dynamic Rule Conflicts and Rule Results in Rule Job Files 25R1.4
Use Case
When conflicting rule results are encountered while running dynamic rules from EDC Tools, the Rules job output file includes new columns with fewer rows to provide more accurate and clear information about what actions took place.
Description
Two new columns, Rule Result and Action Details, are added to the Rules Job preview and job results in the rule_details.csv and create_delete_dynamics_details.csv output files. Conflicting rules are documented in the Action Details column and are no longer shown on separate rows within the file when the conflicting rule was not run as part of the current job.
The Rule Result column displays the result (True or False) of the evaluation of the rule that was run.
The Action Details column displays additional information about the rule action and tells the user what other rules may be overriding the expected action of the rule that was run. The message “Action overridden by existing rule results: ruledef_name” displays when there is another rule that has more specific results acting on the same dynamic target object. There could be one or more rule definitions listed.
Enablement & Configuration
This feature is automatically enabled.
Learn More
Clinical DataBase (CDB) & EDC Clinical Reporting
The following are new features for the Veeva CDB application, EDC Clinical Reporting (the Veeva Clinical Data solution for data cleaning and reporting), or both.
Availability: Clinical DataBase (CDB) is only available to CDB license holders. Contact your Veeva Services representative for details.
Third Party Data Import Enhancements 25R1.3
Use Case
These enhancements to the handling of third party data (3PD) improves ease of use in CDB Workbench.
Description
This release includes two enhancements to third party data import in CDB Workbench:
- For an Open EDC study, when the first package of a 3PD source fails to load, CDB will no longer block data import from any other sources in that study.
- The manifest builder JSON will export in a more human readable format.
Enablement & Configuration
These enhancements are automatically available with the release.
CDB Automatic Table Splitting 25R1.3
Use Case
This is a self-healing operation that previously required a support ticket for Veeva to address.
Description
Large study designs or third party data import packages can cause ingestion failure, due to the size of the table required to store the data. CDB automatically splits these tables, but if a resulting table is still too large, the split size would need to be reduced to handle the large number of columns required. This release enables an automatic process where, if ingestion fails due to the design of the data, the system will retry ingestion with a smaller number of columns in each partial table, allowing ingestion to complete without requiring a Veeva Support ticket.
Enablement & Configuration
This feature is automatically available with the release.
Codelist Support for Third Party Data 25R1.3
Use Case
The ability to define codelist codes and decodes in the manifest allows for better control over reported values in a third party data set or in an OpenEDC study. This also supports transformation of codelist data values.
Description
Codelists for a source can now be defined in the manifest file, ensuring that reported data aligns with the definition of allowed values within that codelist, and transformation of entered data from coded to decoded.
Enablement & Configuration
This feature is automatically available with the release.
Enhanced Conditional Unblinding Rules 25R1.2
Use Case
To support more complex rules for unblinding data, we added additional operators for conditional unblinding rules.
Description
Conditional unblinding rules for text and number items (integer, float) now support the IN and NOT IN operators. This allows rules to easily check if an item’s value matches (or does not match) any value within a single comma-separated list. This enhancement overcomes the previous limitation where a rule could combine a maximum of six (6) distinct conditions or condition groups, as one IN or NOT IN condition can now efficiently test against multiple values simultaneously.
Enablement & Configuration
This feature is automatically available with the release.
Enablement Change: Calculated Fields in SYS Listings 25R1.3
Use Case
These fields provide data that is available in the SDE to be included natively in CDB to be included in exports.
Description
With this release, the Calculated Fields in SYS Listings feature (25R1) is now enabled automatically for all customers. Prior to this release, this feature required enablement by Veeva Support.
When enabled, these fields are applied automatically. This doesn’t cause a change detection for existing Export Definitions.
See the Calculated Fields in SYS Listings release notes for more information.
Enablement & Configuration
This feature is automatically available with the release.
Export: New Columns Added to Raw Core Listings 25R1.2
Use Case
Additional Lab and Coding data offer more details in standard exports.
Description
The standard export now includes the Lab Modifier and Drug Code when using the Raw export type. The Lab Modifier provides, for example, greater than or less than modifiers for lab values. The Drug Code shows as DrugCD in forms that use the WHODrug dictionary for medical coding. These changes will be highlighted with change detection for existing exports.
Enablement & Configuration
This feature is automatically available with the release.
Learn More
Manual Refresh of Non-Production Review Listings 25R1.2 25R1.4
Use Case
To reduce the system processing for test studies that don’t need to be continuously refreshed, we removed the job that runs hourly to refresh review listings. Instead, users can refresh listings on demand in non-production study environments.
Description
This release introduces a new Refresh option for Review Listings in non-production study environments to allow study builders and testers to refresh the listing on demand. This will reduce the burden on the system of continuously running hourly jobs that are not needed, and speed up processing time for testers and study programmers to get a listing refreshed as needed, instead of waiting for the queue to process the listing.
With this release, CDB will no longer reprocess third party data every 24 hours in non-production studies.
Enablement & Configuration
This feature is automatically available with the release.
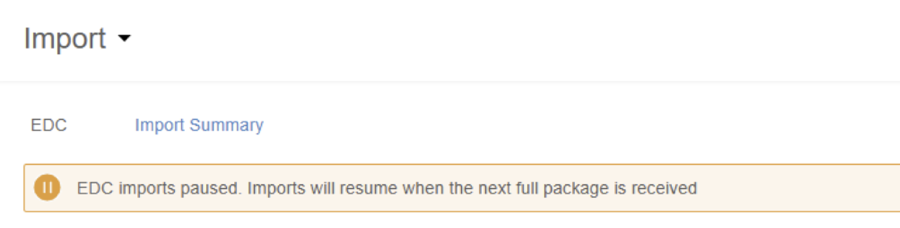
Paused Incremental Ingestion Banner 25R1.3
Use Case
The new banner provides better visibility when incremental ingestion is paused in Clinical Reporting and CDB Workbench.
Description
The Import page in CDB will display a banner when incremental ingestion (the every 15 minute update of data into a study from Veeva EDC) has paused due to a change to the study design or an unexpected load error.
Enablement & Configuration
This feature is automatically available with the release.
SFF API Column Name Deduplication 25R1.3
Use Case
Duplicate column names in the SFF API can cause problems with downstream systems.
Description
When using the Study File Format (SFF) API, if multiple columns have the same definition name, the system appends “_n,” starting with n = 1, for each duplicate column. The first column will maintain the original name, with each successive column being updated with the appended suffix. The manifest file has been updated to reflect this new naming convention.
API versioning and package versioning for SFF will be separate. In 25R2, a new package version, 2.0, will be generated for all extractions.
Enablement & Configuration
This feature is available automatically with the release.
Learn More
Export API: Run and Export Existing Definitions Role Updates 25R1.3
Use Case
API exports offer more flexibility in starting an export job and retrieving the files.
Description
New API calls have been created for both CDB Workbench and Clinical Reporting. These calls allow users to retrieve study information and run and retrieve export definitions via API. Learn more about these new API calls in the Developer Portal.
The CDB API Read Write standard role (available to customers with CDB Workbench) has been updated to include the following permissions:
- View Export
- View Export Packages
- Generate Export Package
- Create Export Definition
- Clinical Reporting Tab
Enablement & Configuration
These new APIs and the automatically assigned permission updates to the CDB API Read Write role are available after the release.
CDB: Maintain Review After Data Change 25R1.3
Use Case
This feature enables Data Managers to know when data in a Review Listing row has been changed after its review status was set. It does this while maintaining the latest review status and comments.
Description
Once a row in a Review Listing has the review status set, if data changes, a delta icon (Δ) will be displayed. The user can then dismiss the delta, retaining the existing status and comments, or update the status as needed. A new column on the review Dashboard will also display these delta changes, showing a count of rows with changes. Review listings with changes will count as not complete in the Review Dashboard and the Clean Patient Tracker.
Review Listings included in an export will include a new column that denotes whether data changed in a previously reviewed row.
Enablement & Configuration
This feature is automatically available with the release.
Learn More
Observations Enhancements 25R1.3
Use Case
These enhancements allow users to be assigned to an observation, new Core Observation listings, and observation CQL syntax.
Description
Users with View All Listings and View Observations permissions can be assigned to an observation. This allows for tracking multiple reviews, facilitating questions between colleagues on a data item, or enabling other forms of collaboration on data review.
Similar to Query listings, new Core Observation listings are now available, including Core listings and observation messages. Within each Core Observation listing, you can filter and sort observations, act on them, and navigate to the Data listing where the observation was created. These Core listings can be downloaded and/or included in export definitions.
You can now use the @OBS and @OBSMSG CQL syntax in Custom listings. Like the @QRY syntax, you can combine the observation syntax with study header data but not form-specific data. Observation summary links within data listings are available. These links navigate users to the All Observations listing, filtered to show observations created from that listing.
Query summary links within data listings are also available. These links navigate users to the All Queries listing filtered for queries generated from that listing.
Enablement & Configuration
These features are automatically available to users with Observations permissions (View, Create, Reply, and Close).
Learn More
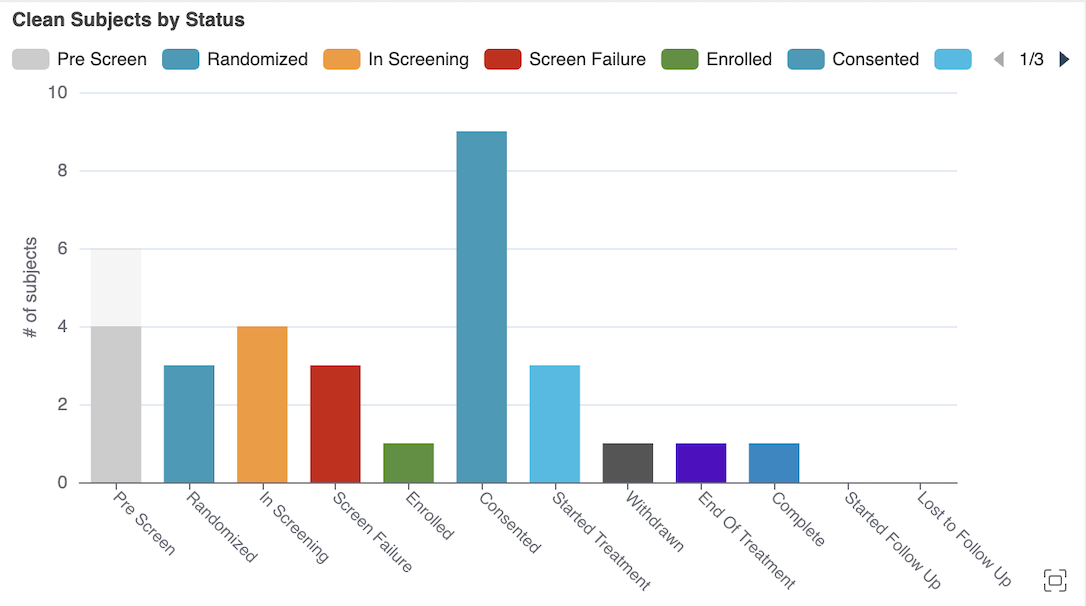
CDB Clean Patient Tracker Site & Subject Charts 25R1.3
Use Case
New charts in the Clean Patient Tracker (Subject page) in CDB Workbench provide more accessible and understandable visualizations of casebooks with remaining data cleaning and overdue data entry. The charts provide a high-level overview, letting Data Managers know and communicate the status of data cleaning and any overdue data entry from the site.
Description
Two new charts in the Clean Patient Tracker show information about data cleaning and data entry for casebooks in the study. A bubble chart shows the relationship between overdue data entry and data cleaning still required. The second chart, a bar graph, shows the number of subjects by status and their cleaning status.
Enablement & Configuration
This feature is automatically available with the release.
Learn More
CDB Quick Queries 25R1.4
Use Case
Quick Queries offers data managers and sites a clean, simple, and automated way to provide standardized query messages. This drastically reduces the keystrokes required from users when updating data and creating and answering queries.
Description
Similar to the update in Veeva EDC, CDB Workbench now supports the creation of Quick Queries, a simple way to denote if a query is intended to confirm the value as entered. This provides site users with a simple response option while also maintaining the ability to include a custom message. Individual and batch query creation supports the quick query option, as well as CDB Checks.
We’ve updated the All Query Messages and EDC Query Messages listings with new columns showing if a query was created using Quick Queries.
Enablement & Configuration
This feature is available when the study configuration is turned on in Studio.
Learn More
Role Management & Security
Features in this section are enhancements to the System Tools > Role Management and System Tools > Users areas, as well as changes to standard Study Roles, security, and access control in Veeva Clinical Data.
Update to CMDS API Read Write and CDMS API Read Only Permissions for Protocol Deviations 25R1.3
Use Case
To prepare for managing Protocol Deviations via the API in a future release, we’re updating permissions for CDMS API users.
Description
The CMDS API Read Write standard role has been updated to include the following permissions:
- View Protocol Deviations
- Create Protocol Deviations
- Edit Protocol Deviations
The CDMS API Read Only standard role has been updated to include the View Protocol Deviations permission.
Enablement & Configuration
The permission updates are automatically assigned to the CMDS API Read Write and CDMS API Read Only roles with the release.
New Vault File Manager Access Permission 25R1.3
Use Case
A new permission “Vault File Manager Access” allows users access to view and manage files within the File Staging tab in the Vault File Manager application.
Description
The permission “Vault File Manager Access” is seen within the Role Management table (Study Administration section) and grants permission to general file staging access, which contains any Extract jobs sent to a vault-level FTP connection. The new permission is added to these standard roles:
- CDMS Super User
- CDMS Lead Data Manager
- CDMS Data Manager
- CDMS User Administrator
- CDMS API Read Write
Enablement & Configuration
This feature is automatically enabled.
Learn More
Connections & Integrations
Features in this section are new connections or integrations with Veeva Clinical Data or enhancements to existing ones.
Safety-EDC Connection: Safety Case Follow-Ups Honor Safety Decisions 25R1.3
Use Case
Via the Safety-EDC Connection, Veeva EDC sends subject data to Veeva Safety not only as part of the actual Safety Case, but also as additional subject information to allow insights beyond the case data. This additional subject information can be added to a Veeva Safety case by simple selection. Subject information can also be removed from the case.
In another scenario, a Safety Case can contain several events when sent from Veeva EDC, which can be split into individual cases on the Safety side, or individual Safety Cases can be merged into one.
Veeva EDC is now being made aware of those safety decisions via the Safety-EDC Connection. While the EDC clinical data and site linking decisions remain unchanged in Veeva EDC, the decision taken in Veeva Safety about adding, removing, spitting or merging will be honored when compiling the next Follow-Up Send.
For example, a concomitant medication may be added in Veeva Safety. This feature guarantees that the Safety Case receives a Follow-Up Send if changes are made to that Concomitant Medication form, although the form isn’t linked by the site in EDC. Prior to this release, there would have been no Follow-Up Send because the form wasn’t linked by the site.
Description
Safety decisions in Veeva Safety include adding and removing additional subject information, or splitting and merging cases. Veeva EDC is notified of these actions and honors the decisions taken in Veeva Safety for smarter case Follow-Up Sends.
This functionality has no impact on the EDC user experience and can’t be tested exclusively in EDC, but requires integrated testing with Veeva Safety. The appropriate Follow-Up creation upon data changes in Veeva EDC and safety decisions in Veeva Safety must be traced in Veeva Safety.
Enablement & Configuration
This feature is automatically enabled for studies using the Safety-EDC Connection.
E2BLink: Include Related Data on Safety Case Initiating Event in Safety Case 25R1.3
Use Case
To allow more flexibility for complex study designs, item groups embedded in the safety case initiating event, usually the Adverse Event EDC form, can now be included in the safety case. These repeating item groups would usually cover study drug dispenses, drug history, in case of death, medical history or test results on the adverse event form, for which no standalone form is set up in the study design. With this ability, relevant information can be entered - as necessary - by a site user when an event will initiate a safety case for the subject.
Description
In Studio > Safety Integrations > Form Configurations, a study designer can now map the EDC form already mapped with the Safety Case Initiation Event data type to other safety data type, including Concomitant Medications, In Case of Death, Medical History, Drug History, Study Drug, or Test Results.
The exception is a second mapping to the Safety Case Initiation Event or Patient Characteristics safety data type. The Reporter / Sender Comments by System data type already support dual usage on the Safety Case Initiation Event data type.
If an EDC form already mapped to the Safety Case Initiation Event data type is being mapped to another safety data type, the Each Entry In field now autoselects Repeating item group in form. Note that the Each Entry In field for the In Case of Death, Patient Characteristics, and Reporter / Sender Comments by System safety data types only allow the Form option for this field.
This new feature is supported by the Copy Forms functionality and reflected in the Study Design Specification (SDS).
Enablement & Configuration
This feature is automatically enabled for studies using the E2BLink.
E2BLink: Study Drug Indication on Study Drug Safety Type 25R1.2
Use Case
The Indication as Reported by the Primary Source (G.k.7.r.1) captures the reporter’s description of the indication for the study drug use. While this might be a single value for all subjects in a study, or per subject, this new option allows the configuration for the indication to vary from dispense to dispense on the main study drug form. If multiple values are used across the subject casebook, all will be included in the E2B XML.
Description
For E2BLink studies, from Studio > Form Configurations > Item Configuration, users can now map G.k.7.r.1 (Indication as Reported by the Primary Source) to an Item Definition on the Study Drug safety data type.
Enablement & Configuration
This feature is automatically enabled for studies using the E2BLink. Configuration for a live study might result in follow-up safety messages for existing safety cases.
Safety Integration: Inclusion of Secondary Event Site Links into the Safety Case 25R1.3
Use Case
Safety cases are initiated by the primary Safety Case Initiating Event, which is most commonly a serious adverse event. Based on the inclusion rules in the safety integration setup in Veeva EDC, other secondary events, which are typically non-serious adverse events, can be included in the case. These events can be linked by the site to additional safety case relevant information, including concomitant medication and medical history. While this linked information could always be included in the safety case for the primary initiating event, this information can now also be included for secondary events that are part of the safety case.
Description
This new study setting is available for both the Safety-EDC Connection and the E2BLink.
In Studio > Safety Integration > Safety Settings, users are now able to configure the study to Include Secondary Event Site Links to Case.
Setting this field to Yes results in the inclusion of all configured data from forms that are linked to any secondary event into the case. Note that this is set to Yes by default in all new Studies.
For the Safety-EDC Connection, all unlinked safety-relevant data will still be transferred to Veeva Safety and made available as additional subject information, beyond the new information now included in the actual safety case.
As part of this feature, we renamed the Safety Form Type label in Safety Integration > Form Configuration to Safety Data Type to better reflect the type of data transfer.
Enablement & Configuration
This feature is automatically enabled for studies using the Safety-EDC Connection or E2BLink. Configuration to include secondary event site links for a live study might result in follow-up safety messages for existing safety cases.
Clinical Operations-EDC Connection: Send Event Group Definition-Event Definition Relationships to CTMS 25R1.3
Use Case
Currently, the Clinical Operations - EDC Connection automatically transfers Visit Group Definitions and Visit Definitions from Veeva EDC to Veeva CTMS but does not transfer the relationships between them. This feature enhances the Clinical Operations - EDC Connection by enabling it to create and manage relationships between Visit Group Definitions and Visit Definitions in Veeva CTMS. It bases these relationships on the corresponding Event Group Definition - Event Definition objects in EDC. This enhancement provides a more detailed and accurate representation of study visit structures in Veeva CTMS, leading to better management and alignment with study protocols.
Description
If this feature is configured for the Clinical Operations - EDC Connection, for each related Event Group Definition - Event Definition record found in EDC, the system will either update an existing record in CTMS or create a new one. If a matching Visit Group Definition or Visit Definition is missing in CTMS, a user exception message is generated.
This functionality has no impact on the EDC user experience and cannot be tested in EDC. On the CTMS side, this feature enables Veeva CTMS to display Visit Group Definition - Visit Definition relationships.
Enablement & Configuration
In EDC, this feature is automatically enabled for studies using the Clinical Operations - EDC Connection. In CTMS, the new integration point must be activated to enable the feature.
Clinical Operations-EDC Connection: Transfer of Closeout PDFs to Veeva Clinical Operations 25R1.3
Use Case
This automation streamlines the process of transferring Closeout PDFs to the Veeva eTMF and sites for archiving purposes by not only reducing the manual effort needed to transfer the individual documents, but also automatically storing and updating the Detail PDFs as one document per subject in Veeva eTMF.
Description
When closing out a site in a study, Veeva EDC allows a Closeout PDF containing Detail PDFs to be generated for each subject at the site. Previously the Closeout PDF could only be downloaded from Veeva EDC for archiving. With this release, Closeout PDFs can also be automatically transferred to Veeva eTMF via the Clinical Operations – EDC Connection and shared with sites via SiteConnect. Re-generating the Closeout PDF in Veeva EDC will automatically update the relevant documents in Veeva eTMF via the Clinical Operations – EDC Connection.
Once this feature is configured for the Clinical Operations – EDC Connection, every Closeout PDF generated for a study that uses the Connection will be automatically transferred to Veeva eTMF. Detail PDFs will be ingested by Veeva eTMF as individual documents, as configured. If the Closeout PDF generation fails, for example, if the Site Closeout is performed for a site without subjects, no PDF will be transferred.
As part of this implementation in EDC Tools > Study Settings, the labels for the following fields have been updated:
- “Connect to Vault CTMS” to “Connect to Clinical Operations Vault”
- “CTMS Study Link ID” to “Clinical Operations Study Link ID”.
This functionality has no impact on the EDC user experience and can’t be tested in EDC. The appropriate Closeout PDF transfer must be traced in Veeva Clinical Operations.
Enablement & Configuration
This feature is automatically enabled for studies using the Clinical Operations – EDC Connection. The feature requires configuration in Veeva Clinical Operations.
Learn More
EDC API
The following are new features for the EDC API. See our Developer Portal's release notes for more detailed feature information.
EDC API Features 25R1.4
This release includes the following features for EDC developers:
- Study Country Optional for All Endpoints
- Start Job for S3 Connection and Coding Audit Trail Support
- Casebook Creation Controlled by Licensing and Billing Status
CDB API
The following are new features for the CDB API. See our Developer Portal's release notes for more detailed feature information.
CDB API Features 25R1.3
This release includes the following features for CDB developers:
- Study File Format (SFF) API Update: Deduplication of CSV Columns
- Export API: Run and Export Existing Definitions
EDC Migrator
Features in this section are new features for Veeva EDC Migrator.
Migration Vault Renamed to “Veeva EDC Migrator” 25R1.3
Use Case
For clarity and simplicity, we’ve updated the name of the system. This change also ensures consistency across Veeva applications and aligns with the 25R1 Name & Logo Changes feature.
Description
The Migration Vault system logo will be updated to display the name “Veeva EDC Migrator”.
Enablement & Configuration
This feature is automatically available with the release.
Automated Update Outdated Normals Job 25R1.3
Use Case
During study migration, any change that impacts lab normals (such as the recalculation of a missing subject’s age) previously required the Update Outdated Normals job to be run manually. This release automates that step.
Description
New functionality is now available to recalculate missing subject ages and run the Update Outdated Normals job, eliminating the need for manual intervention in these tasks.
Enablement & Configuration
This feature is immediately available for new migrations.
YAML Builder & Mapping Enhancements 25R1.3
Use Case
These updates add clarity within the YAML builder and its files and minimize the need for manual mapping.
Description
This release includes several enhancements:
-
Unit Item Definitions: Migration Vault now supports mapping Unit Item Definitions in the Form’s YAML and sending item unit data.
-
Lab Codelist Value Mapping: Prior to 25R2, lab codelists required manual mapping. With this release, the YAML Builder now supports the mapping of these items.
-
Rename YAML File for Log Events: Previously the file created by the YAML Builder was named events_unscheduled.yaml. It will now be named events_log.yaml.
-
YAML Builder: Within the Data Source option, “CDMS” has been renamed to “Veeva.”
Enablement & Configuration
These updates are immediately available.