Study Data Extracts
25R3 Version
The Study Data Extract (SDE) job allows data managers to extract and download study execution data from EDC Tools. You can choose to download your data as a CSV, SAS, or XPT file before running the job. Note that the encoding for CSVs and SAS is UTF-8, so it is recommended that you set your encoding to UTF-8 when viewing the export. This page shows SDE columns for the current release. To view columns from previous versions, see SDE Versioning.
Special Characters: If a study name has special characters, those characters will be changed to underscores when downloaded as an SAS file. If a dataset name begins with a number, Vault will add an underscore to the beginning of the file name for that dataset when downloaded as an SAS file.
Repeating Item Groups: Vault adds a row of data for each repeating Item Group. Rows are distinguished by their Item Group Sequence.
Timezones: Vault uses the UTC timezone, Site’s timezone, and user’s timezone across all datasets, which are displayed as separate columns for clinical datetime items. System datetime columns (like LASTRUN) use the UTC timezone. Note that the Job History tab will display the file created in the user’s timezone while files on the user’s computer may display in UTC timezone, depending on the user’s Operating System. Learn more about timezones here.
Prerequisites
Users with the standard CDMS Lead Data Manager study role can perform the actions described above by default. If your vault uses custom Study Roles, your role must have the following permissions:
| Type | Permission Label | Controls |
|---|---|---|
| Standard Tab | EDC Tools Tab | Ability to access the EDC Tools tab |
| Functional Permission | Manage Jobs | Ability to create, edit, and delete scheduled jobs |
| Functional Permission | View Casebook | Ability to view information about and from subject Casebooks (for reports, dashboards, and CDB) |
| Functional Permission | API Access | Ability to access and use the Vault EDC API. (This permission is also required to use CDB.) |
If your Study contains restricted data, you must have the Restricted Data Access permission to view it.
Learn more about Study Roles.
Clinical Datasets
Each clinical dataset, and each Form (Adverse Events, Concomitant Medications, etc.), contains a set of Key Columns and a set of Clinical Data columns, which are detailed below.
Standard Clinical Dates & Datetimes
We support the following standard date and datetime formats for system-generated values across SDE versions:
- Dates: YYYY-MM-DD
- Datetimes: yyyy-MM-dd’T’HH:mmZ
Dates and Datetimes that aren’t raw values will follow the above format.
Raw Clinical Dates & Datetimes (Version 24R3 and later)
When running the 24R3 version (and any later versions) of the SDE, there will be a RAW column appended to the _Item Definition Name in the column or, if applicable, the Item’s External ID. The _RAW column shows the original date and datetime format value as entered by the site in Data Entry.
For example, the _RAW column would display as shown below:
| aestart | 2024-07-01T12:00-07:00 |
|---|---|
| aestart_UTC | 2024-07-01T19:00Z |
| aestart_USER | 2024-07-01T12:00-07:00 |
| aestart_RAW | 01-Jul-2024 12:00 |
An unknown date would display as the following:
| aestart | 2024-UN-UN |
|---|---|
| aestart_RAW | UN-UN-2024 |
Key Columns
Key Columns contain data to help identify which Form each row is referring to (ex: which Subject, Site, Cycle, or Event that particular form is located in).
The list of Key Columns is as follows:
| Column Header | Label | Type | SAS Type | SAS Informat | SAS Format | Length | SAS Length | Notes |
|---|---|---|---|---|---|---|---|---|
| STUDYID | Study | Text | Char | $ | 128 | $512 | This column refers to study_label or study in the API. | |
| COUNTRY | Country | Text | Char | $ | 128 | $512 | ||
| SITENUM | Study Site Number | Text | Char | $ | 128 | $512 | ||
| SUBJID | Subject | Text | Char | $ | 128 | $512 | ||
| EGROUP | Event Group | Text | Char | $ | 128 | $512 | ||
| EGROUPDEF | Event Group Definition | Text | Char | $ | 128 | $512 | ||
| EGROUPEID | Event Group External ID | Text | Char | 128 | 512 | |||
| EVENT | Event | Text | Char | $ | 128 | $512 | ||
| EVENTDEF | Event Definition | Text | Char | $ | 128 | $512 | ||
| EVENTEID | Event External ID | Text | Char | $ | 128 | $512 | ||
| EVENTDT | Event Date | Date | Num | : vdate. | DATE9. | 14 | 8 | |
| VISMETHOD | Visit Method | Text | Char | $ | 100 | $400 | This column is only visible if Visit Method is enabled. | |
| ESEQ | Event Group Sequence | Integer | Num | 14 | 8 | |||
| FORM | Form | Text | Char | $ | 128 | $512 | ||
| FORMDEF | Form Definition | Text | Char | $ | 128 | $512 | ||
| FORMEID | Form External ID | Text | Char | $ | 128 | $512 | ||
| FSEQ | Form Sequence | Integer | Num | 14 | 8 | |||
| FORMSTATUS | Form Status | Text | Char | 100 | 400 | |||
| CREATEDT | Datetime Form Created | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| FIRSTSUBMITDT | Datetime Form First Submitted | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | This field will only be populated for new studies created after 23R3. |
| LASTSUBMITDT | Datetime Form Last Submitted | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| IGSEQ | Item Group Sequence | Integer | Num | 14 | 8 | |||
| DLASTMOD | Datetime of last data change (UTC). This field is derived by taking the most recent datetime from all of the items' value_modified_date__v on the form. | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | Datetime columns can be separated into two separate Date and Time columns. |
| FGUID | Internal Vault ID (Forms) | Text | Char | $ | 100 | $400 | For HVO objects, the Length is 15, SAS Length is 60 | |
| IGGUID | Internal Vault ID (item groups) | Text | Char | $ | 100 | $400 | For HVO objects, the Length is 15, SAS Length is 60 | |
| FORMILB | Intentionally Left Blank | Boolean | Char | $ | 5 | $20 | ||
| LASTRUN | Last run of this data/listing | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| (Form Link ItemDef Name)_DEF | [Form Link Item Label] Definition | Text | Char | $ | 200 | $800 | If Item to Form Linking is enabled. Format of the cell value: Form Label that is linked to the item. The Form Sequence (#X) is included if the form is repeating. | |
| (Form Link ItemDef Name)_LINKEDFORMID | [Form Link Item Label] Linked Form ID | Text | Char | $ | 100 | $400 | If Item to Form Linking is enabled. | |
| (Form Link ItemDef Name)_LINKID | [Form Link Item Label] Link ID | Text | Char | $ | 100 | $400 | If Item to Form Linking is enabled. | |
| LINKEDTO 1 | Links to other forms | Text | Char | $ | 1500 | $6000 | If Item to Form Linking is enabled. | |
| ITEMLINKEDTO 2 | Item Linked To | Text | Char | $ | 1500 | $6000 | If Item to Form Linking is enabled. | |
| LINKEDITEM | Linked Item | Text | Char | $ | 1500 | $6000 | If Item to Form Linking is enabled. | |
| (ITEMNAME) | (Item Label) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| (ITEMNAME)_UTC | (Item Label)_UTC | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| (ITEMNAME)_USER | (Item Label)_User | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
|
1 This column displays in the following format: 2 This column displays in the following format: |
||||||||
This format shows up to 3 items on the form, up to the maximum character limit.
Clinical Data
After the Key Columns, the system will insert Clinical Data columns, with each column representing an item on the Form.
Clinical Data Item Headers use the item names or external ID defined in Studio. Some items can be associated with more than one column, depending on their data type:
- Codelists are split into two columns:
- ITEMNAME: this column contains the codelist code
- ITEMNAME_D: this column contains the codelist decode or label
- Units are split into four columns:
- ITEMNAME: this column contains the value entered in the item
- ITEMNAME_U: this column contains the unit entered in the item
- ITEMNAME_TRANS: this column contains the “translated” item value, which is the value that was entered that has been converted to the standard unit
- ITEMNAME_TRANSU: this column contains the standard unit
- Datetimes are split into three columns:
- ITEMNAME: this column contains the datetime value entered (site’s timezone)
- Format example: 2024-09-04T12:00+02:00
- ITEMNAME_UTC: this column contains the datetime value converted to UTC
- Format example: 2024-09-04T10:00Z
- ITEMNAME_USER: this column contains the datetime value converted to the timezone of the user running the Study Data Extract
- Format example: 2024-09-04T03:00-07:00
- ITEMNAME: this column contains the datetime value entered (site’s timezone)
Unknown parts in dates and datetimes are represented by UN in ISO format:
- For a date: 2021-UN-UN
- For a datetime: 2021-UN-TUN:UN:UN.UNKZ
You can choose from the following Clinical Data options:
| Option | Description |
|---|---|
| Use Item External ID instead of Item Name for column headers | Replaces the column headers in the clinical datasets (which currently use the Item Name) with the Item External ID configured in Studio |
| Include Separate Date and Time columns for Datetime items | Creates two additional columns with the postfixes (Item)_DT and (Item)_TM for the Date and Time of the Datetime item |
| Include forms Intentionally Left Blank | Creates a column in the clinical datasets called FORMILB that indicates whether the form has been marked Intentionally Left Blank in clinical datasets (default behavior is to skip rows) |
| Exclude blank forms | Excludes forms whose status = "Blank" in the clinical datasets. Note that these forms will still appear in the SYS_FORM dataset |
Casebook Versions
All casebook versions are displayed in Study Data Extracts, so items that were deleted from the schedule in subsequent casebook versions will still appear in Clinical Datasets. This inclusion guarantees that all data is extracted, even if certain subjects were kept on a previous casebook version.
The Study Data Extract job first adds columns for all items that are present in Casebook Version 1 before adding columns for any items that may have been added in subsequent casebook versions to create the Study Data Extract file.
Lab Columns
Each form configured with Local Labs contains certain columns that are dynamically added after the LASTRUN column for lab items. Refer to the table below for a complete list of columns.
23R1 Version Lab Format Change: Clinical forms that are configured with Local Labs are formatted to decrease the number of columns outputted in SDE Version 23R1. In this version, Labs columns are shared across all analytes on the form instead of using multiple columns per analyte. See the [SDE Versioning page]({{ ‘/study-administrators/sde-versioning/’ | prepend: site.baseurl }}) for the list of Labs columns included in previous SDE versions.
In SDE versions prior to 23R1, lab columns that are related to Codelist & Text type analytes may contain mismatches between the lengths for analyte-specific labs columns in the Analyte Library and the Item Definition defined in Studio. We recommend keeping this in mind when adjusting analyte lengths in the Analyte Library in order to prevent data truncation in extracts.
| Column Header | Label | Type | SAS Type | SAS Informat | SAS Format | Length | SAS Length | Notes |
|---|---|---|---|---|---|---|---|---|
| STUDYID | Study | Text | Char | $ | 128 | $512 | ||
| COUNTRY | Country | Text | Char | $ | 128 | $512 | ||
| SITENUM | Study Site Number | Text | Char | $ | 128 | $512 | ||
| SUBJID | Subject | Text | Char | 128 | $512 | |||
| EGROUP | Event Group | Text | Char | $ | 128 | $512 | ||
| EGROUPDEF | Event Group Definition | Text | Char | $ | 128 | $512 | ||
| EGROUPEID | Event Group External ID | Text | Char | $ | 128 | $512 | ||
| EVENT | Event | Text | Char | $ | 128 | $512 | ||
| EVENTDEF | Event Definition | Text | Char | $ | 128 | $512 | ||
| EVENTEID | Event External ID | Text | Char | $ | 128 | $512 | ||
| EVENTDT | Event Date | Date | Num | :vdate. | DATE9. | 14 | 8 | |
| ESEQ | Event Group Sequence | Integer | Num | 14 | 8 | |||
| FORM | Form | Text | Char | $ | 128 | $512 | ||
| FORMDEF | Form Definition | Text | Char | $ | 128 | $512 | ||
| FORMEID | Form External ID | Text | Char | $ | 128 | $512 | ||
| FSEQ | Form Sequence | Integer | Num | 14 | 8 | |||
| IGROUP | Item Group | Text | Char | $ | 500 | $2000 | ||
| IGROUPDEF | Item Group Definition | Text | Char | $ | 128 | $512 | ||
| IGROUPEID | Item Group External ID | Text | Char | $ | 128 | $512 | ||
| IGSEQ | Item Group Sequence | Integer | Num | 14 | 8 | |||
| DLASTMOD | Datetime of last data change (UTC) | Datetime | Num | :vdatetime. | DATETIME22.3 | 14 | 8 | |
| FGUID | Internal Vault ID (Forms) | Text | Char | $ | 100 | $400 | For HVO objects, the Length is 15, SAS Length is 60 | |
| IGGUID | Internal Vault ID (Item Groups) | Text | Char | $ | 15 | $60 | For HVO objects, the Length is 15, SAS Length is 60 | |
| LASTRUN | Last run of this data/listing | Datetime | Num | :vdatetime. | DATETIME22.3 | 14 | 8 | |
| LBDTC | Collection Date Time | Datetime | Num | DATETIME22.3 | 14 | 8 | ||
| LBDTC_UTC | Collection Date Time_UTC | Datetime | Num | DATETIME22.3 | 14 | 8 | ||
| LBDTC_USER | Collection Date Time_USER | Datetime | Num | DATETIME22.3 | 14 | 8 | ||
| LBDTC_RAW | Collection Datetime_RAW | Datetime | Char | $ | 64 | $256 | ||
| LBLOC | Lab Location* | Text | Char | $ | 128 | $512 | ||
| LBAGE | Age | Integer | Num | 14 | 8 | |||
| LBAGE_U | Age_U | Text | Char | 128 | $512 | |||
| LBAGE_TRANS | Age_TRANS | Integer | Num | 14 | 8 | |||
| LBAGE_TRANSU | Age_TRANSU | Text | Char | $ | 128 | $512 | ||
| LBFAST | Fasting Status | Text | Char | $ | Based on Item Definition | Based on Item Definition x4 | ||
| LBFAST_D | Fasting Status_D | Text | Char | $ | 256 | $1024 | ||
| LBFEMALECYCLE | Female Cycle | Text | Char | $ | Based on Item Definition | Based on Item Definition x4 | ||
| LBFEMALECYCLE_D | Female Cycle_D | Text | Char | $ | 256 | $1024 | ||
| LBTEST | Lab Test | Text | Char | $ | 128 | $512 | Lab Analyte Definition Label | |
| LABMODIFIER | Lab Modifier | Text | Char | $ | 128 | $512 | Visible for Studies using Global (Versionless) Labs if at least one analyte definition has Lab Modifiers configured. | |
| LABMODIFIER_D | Lab Modifier_D | Text | Char | $ | 256 | $1024 | Visible for Studies using Global (Versionless) Labs if at least one analyte definition has Lab Modifiers configured. | |
| LBORRES | Lab Result | Text, Float, or Integer | Char or Num | $ | 1500 or 14 | $6000 or 8 | Data type may be float or integer if all cells in that column have the same data type. Note that if the data type is float or integer, the length/SAS length may change to the default for numeric values. | |
| LBORRES_U_D | Lab Result Unit or Decode for Codelists and Units | Text | Char | $ | 1500 | $6000 | Visible for Unit, Number, Codelist, or Text analyte type. | |
| LBORRES_TRANS | Lab Result_TRANS | Float or Integer | Num | 14 | 8 | Data type may be integer if all cells in that column are integers. | ||
| LBORRES_TRANSU | Lab Result_TRANSU | Text | Char | $ | 1500 | $6000 | ||
| LBORNRLO | Normal Range Lower Limit | Float or Integer | Num | 14 | 8 | Data type may be integer if all cells in that column are integers. | ||
| LBORNRHI | Normal Range Upper Limit | Float or Integer | Num | 14 | 8 | Data type may be integer if all cells in that column are integers. | ||
| LBORNRLOHI_U | Normal Range Lower and Upper Limit_U | Text | Char | $ | 1500 | $6000 | ||
| LBORNRLO_TRANS | Normal Range Lower Limit_TRANS | Float or Integer | Num | 14 | 8 | Data type may be integer if all cells in that column are integers. | ||
| LBORNRHI_TRANS | Normal Range Upper Limit_TRANS | Float or Integer | Num | 14 | 8 | Data type may be integer if all cells in that column are integers. | ||
| LBORNRLOHI_TRANSU | Normal Range Lower and Upper Limit_TRANSU | Text | Char | $ | 1500 | $6000 | ||
| LBOVRDNRLO | Normal Range Override Lower Limit | Float or Integer | Num | 14 | 8 | Data type may be integer if all cells in that column are integers. | ||
| LBOVRDNRHI | Normal Range Override Upper Limit | Float or Integer | Num | 14 | 8 | Data type may be integer if all cells in that column are integers. | ||
| LBOVRDNRLOHI_U | Normal Range Override Lower and Upper Limit_U | Text | Char | $ | 1500 | $6000 | ||
| LBOVRDNRLO_TRANS | Normal Range Override Lower Limit_TRANS | Float or Integer | Num | 14 | 8 | Data type may be integer if all cells in that column are integers. | ||
| LBOVRDRLOHI_TRANSU | Normal Range Override Lower and Upper Limit_TRANSU | Text | Char | $ | 1500 | $6000 | Data type may be integer if all cells in that column are integers. | |
| LBSTNRC | Normal Value | Text | Char | $ | 1500 | $6000 | For Codelist or Text-type Analytes. | |
| LBSTNRC_D | Normal Value_D | Text | Char | $ | 256 | $1024 | For Codelist-type Analytes. | |
| LBOVRDNRC | Normal Value Override | Text | Char | $ | 1500 | $6000 | For Codelist or Text-type Analytes. | |
| LBOVRDNRC_D | Normal Value Override_D | Text | Char | $ | 256 | $1024 | For Codelist-type Analytes. | |
| LBNRIND | Normal Range Indicator | Text | Char | $ | 1500 | $6000 | ||
| LBCLSIG | Clinical Significance | Text | Char | $ | 1500 | $6000 |
*This field refers to the Lab ID.
The LBDTC, LBLOC, and LBAGE columns are part of the Lab Header that is generated for each lab form. Each column related to a Lab Analyte with a “Unit” type has additional columns for the Unit (appended with _U), the Translated Value (appended with _TRANS), and the Translated Unit (appended with _TRANSU). For example, the Lab Result Unit for Sodium would display in the column header as LBORRES_Sodium_U.
Coding Data
If a form is set up for coding, meaning that a coding configuration has been defined in Studio, coding-related columns will be added after clinical data columns. If there are multiple verbatims set up in a form, the system will add a set of coding columns for each verbatim configured. If the same item in different item groups is configured for coding, the system will add “itemgroup.item” to each column header to indicate which set of columns belongs to which verbatim. Note that you need the View Code permission to view coding columns in the SDE.
List of coding columns for MedDRA:
| Column Header | Label | Type | SAS Type | SAS Informat | SAS Format | Length | SAS Length |
|---|---|---|---|---|---|---|---|
| CRSTATUS | Coding Status | Text | Char | $ | 100 | $400 | |
| DICTTYPE | Dictionary Type | Text | Char | $ | 100 | $400 | |
| DICTVER | Dictionary Release | Text | Char | $ | 128 | $512 | |
| SOC | SOC | Text | Char | $ | 1500 | $6000 | |
| SOCID | SOC Code | Text | Char | $ | 1500 | $6000 | |
| HLGT | HLGT | Text | Char | $ | 1500 | $6000 | |
| HLGTID | HLGT Code | Text | Char | $ | 1500 | $6000 | |
| HLT | HLT | Text | Char | $ | 1500 | $6000 | |
| HLTID | HLT Code | Text | Char | $ | 1500 | $6000 | |
| PT | PT | Text | Char | $ | 1500 | $6000 | |
| PTID | PT Code | Text | Char | $ | 1500 | $6000 | |
| LLT | LLT | Text | Char | $ | 1500 | $6000 | |
| LLTID | LLT Code | Text | Char | $ | 1500 | $6000 | |
| PRIMPATH | Primary Path | Text | Char | $ | 1500 | $6000 | |
| LASTCODEDAT | Last Coded Date | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 |
| LASTCODEDBY | Last Coded By | Text | Char | $ | 201 | $804 |
List of coding columns for WHODrug:
| Column Header | Label | Type | SAS Type | SAS Informat | SAS Format | Length | SAS Length |
|---|---|---|---|---|---|---|---|
| CRSTATUS | Coding Status | Text | Char | $ | 100 | $400 | |
| DICTTYPE | Dictionary Type | Text | Char | $ | 100 | $400 | |
| DICTVER | Dictionary Release | Text | Char | $ | 128 | $512 | |
| ATC1 | ATC1 | Text | Char | $ | 1500 | $6000 | |
| ATC1CD | ATC1 Code | Text | Char | $ | 1500 | $6000 | |
| ATC2 | ATC2 | Text | Char | $ | 1500 | $6000 | |
| ATC2CD | ATC2 Code | Text | Char | $ | 1500 | $6000 | |
| ATC3 | ATC3 | Text | Char | $ | 1500 | $6000 | |
| ATC3CD | ATC3 Code | Text | Char | $ | 1500 | $6000 | |
| ATC4 | ATC4 | Text | Char | $ | 1500 | $6000 | |
| ATC4CD | ATC4 Code | Text | Char | $ | 1500 | $6000 | |
| PREFNAME | Preferred Name | Text | Char | $ | 1500 | $6000 | |
| PREFCODE | Preferred Code | Text | Char | $ | 1500 | $6000 | |
| DRUGNAME | Drug Name | Text | Char | $ | 1500 | $6000 | |
| DRUGCODE | Drug Code | Text | Char | $ | 1500 | $6000 | |
| PREFBASE | Preferred Base Code | Text | Char | $ | 1500 | $6000 | |
| PREFLABEL | Preferred Base | Text | Char | $ | 1500 | $6000 | |
| LASTCODEDAT | Last Coded Date | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 |
| LASTCODEDBY | Last Coded By | Text | Char | $ | 201 | $804 |
List of coding columns for JDrug:
| Column Header | Label | Type | SAS Type | SAS Informat | SAS Format | Length | SAS Length |
|---|---|---|---|---|---|---|---|
| CRSTATUS | Coding Status | Text | Char | $ | 100 | $400 | |
| DICTTYPE | Dictionary Type | Text | Char | $ | 100 | $400 | |
| DICTVER | Dictionary Release | Text | Char | $ | 128 | $512 | |
| DRUGCODE | Drug Code | Text | Char | $ | 1500 | $6000 | |
| DRUGNAME | Drug Name | Text | Char | $ | 1500 | $6000 | |
| GDRUGNAME | Generic Drug Name | Text | Char | $ | 1500 | $6000 | |
| DRUGCODECAT1 | Drug Code Category 1 | Text | Char | $ | 1500 | $6000 | |
| DRUGCODECAT2 | Drug Code Category 2 | Text | Char | $ | 1500 | $6000 | |
| USECAT1 | Use Category 1 | Text | Char | $ | 1500 | $6000 | |
| USECAT2 | Use Category 2 | Text | Char | $ | 1500 | $6000 | |
| MANNAME | Manufacturer Name | Text | Char | $ | 1500 | $6000 | |
| MANCODE | Manufacturer Code | Text | Char | $ | 1500 | $6000 | |
| MAINTFLG | Maintenance Flag | Text | Char | $ | 1500 | $6000 | |
| MAINTDT | Maintenance Date | Text | Char | $ | 1500 | $6000 | |
| LASTCODEDAT | Last Coded Date | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 |
| LASTCODEDBY | Last Coded By | Text | Char | $ | 201 | $804 |
Definitions File
The Study Data Extract ZIP file contains a definition file, which is a listing of all columns across all datasets included in the extract.
List of columns included in the Definitions file:
| Column Header | Data |
|---|---|
| Dataset Name | Name of the dataset |
| Column | Name of the column |
| Type | Item type for clinical data items |
| SAS Type | SAS type of the column |
| SAS Informat | SAS informat applied |
| SAS Format | SAS format applied |
| Length | Length in characters |
| SAS Length | SAS length in bytes |
| Label | SAS Label of the column |
Length standardization: As of the 22R3 release, the SDE uses Vault and Studio lengths when possible. If either the Vault or Studio length changes, it will impact the length shown in the Definitions file for CSV and SAS as well as the SAS file itself. Vault length can change due to HVO, Data Model, or other infrastructure changes.
System Datasets
Along with clinical datasets, the Study Data Extract file contains several system datasets that contain operational data and metrics.
LBFAST and Timezone Columns: The LBFAST column and Timezone column will be available in the SYS_LABRANGES and SYS_LABLOC SDE datasets, respectively, in a future release to support Labs Fasting Status and Timezone collection.
SYS_SITE
The SYS_SITE dataset lists all sites for the selected study.
List of SYS_SITE columns:
| Column Header | Label | Type | SAS Type | SAS Informat | SAS Format | Length | SAS Length | Notes |
|---|---|---|---|---|---|---|---|---|
| STUDYID | Study | Text | Char | $ | 128 | $512 | ||
| COUNTRY | Study Country | Text | Char | $ | 128 | $512 | ||
| SITENUM | Study Site Number | Text | Char | $ | 128 | $512 | ||
| SITENAME | Study Site Name | Text | Char | $ | 128 | $512 | ||
| INVNAME | Principal Investigator | Text | Char | $ | 128 | $512 | ||
| CASEBDEF | Casebook Version | Integer | Num | 14 | 8 | |||
| STATUS | Site Status | Text | Char | $ | 100 | $400 | ||
| TIMEZONE | Timezone of the Site | Text | Char | $ | 100 | $400 | ||
| BULKCASEBOOKSIGNATURE | Bulk Casebook Signature | Boolean | Char | $ | 5 | $20.00 | ||
| GUID | Internal Vault ID of the site | Text | Char | $ | 100 | $400 | For HVO objects, the Length is 15, SAS Length is 60 | |
| LASTRUN | Last run of this data/listing | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 |
SYS_SUB
The SYS_SUB dataset lists all subjects for the selected study.
List of SYS_SUB columns:
| Column Header | Label | Type | SAS Type | SAS Informat | SAS Format | Length | SAS Length | Notes |
|---|---|---|---|---|---|---|---|---|
| STUDYID | Study | Text | Char | $ | 128 | $512 | ||
| COUNTRY | Country | Text | Char | $ | 128 | $512 | ||
| SITENUM | Study Site Number | Text | Char | $ | 128 | $512 | ||
| SUBJID | Subject | Text | Char | $ | 128 | $512 | ||
| IXRSID | Subject IXRS ID | Text | Char | $ | 255 | $1020 | This column refers to an external Subject ID that may be set through API or Integration. | |
| CASEBDEF | Casebook Version | Integer | Num | 14 | 8 | |||
| STATUS | Status of the Subject/Casebook | Text | Char | $ | 100 | $400 | ||
| SDVPLAN | SDV Plan | Text | Char | $ | 128 | $512 | ||
| DMRPLAN | DMR Plan | Text | Char | $ | 128 | $512 | ||
| FROZEN | Subject Frozen | Boolean | Char | $ | 5 | $20 | Populated for Data Model 2 studies only. | |
| LOCKED | Subject Locked | Boolean | Char | $ | 5 | $20 | Populated for Data Model 2 studies only. | |
| SIGNED | Subject Signed | Boolean | Char | $ | 5 | $20 | Populated for Data Model 2 studies only. | |
| GUID | Internal Vault ID of the subject | Text | Char | $ | 100 | $400 | For HVO objects, the Length is 15, SAS Length is 60 | |
| LASTMODDT | Subject Last Modified Datetime | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| LASTRUN | Last run of this data/listing | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| LATESTARM | Latest Arm | Text | Char | $ | 128 | $512 | ||
| LATESTCOHORT | Latest Cohort | Text | Char | $ | 128 | $512 | ||
| LATEST SUBSTUDY | Latest Substudy | Text | Char | $ | 128 | $512 | ||
| CNSNTDT | Initial Consent Date | Date | Num | : vdate. | DATE9. | 14 | 8 | |
| SCRDDT | Screened date | Date | Num | : vdate. | DATE9. | 14 | 8 | |
| SCRFAILDT | Screen failed date | Date | Num | : vdate. | DATE9. | 14 | 8 | |
| ENRDDT | Enrolled date | Date | Num | : vdate. | DATE9. | 14 | 8 | |
| RDMDDT | Randomized date | Date | Num | : vdate. | DATE9. | 14 | 8 | |
| STARTTRTDT | Started Treatment Date | Date | Num | : vdate. | DATE9. | 14 | 8 | |
| ENDTRTDT | End of treatment date | Date | Num | : vdate. | DATE9. | 14 | 8 | |
| WTHDRWNDT | Withdrawn date | Date | Num | : vdate. | DATE9. | 14 | 8 | |
| STARTFLLWUPDT | Started Follow Up Date | Date | Num | : vdate. | DATE9. | 14 | 8 | |
| LOSTFLLWUPDT | Lost to Follow Up Date | Date | Num | : vdate. | DATE9. | 14 | 8 | |
| CMPLTDT | End of study date | Date | Num | : vdate. | DATE9. | 14 | 8 |
SYS_EVT
The SYS_EVT dataset lists all events as well as event dates and status for the selected study.
List of SYS_EVT columns:
| Column Header | Label | Type | SAS Type | SAS Informat | SAS Format | Length | SAS Length | Notes |
|---|---|---|---|---|---|---|---|---|
| STUDYID | Study | Text | Char | $ | 128 | $512 | ||
| COUNTRY | Country | Text | Char | $ | 128 | $512 | ||
| SITENUM | Study Site Number | Text | Char | $ | 128 | $512 | ||
| SUBJID | Subject | Text | Char | $ | 128 | $512 | ||
| EGROUP | Event Group | Text | Char | $ | 128 | $512 | ||
| EGROUPDEF | Event Group Definition | Text | Char | $ | 128 | $512 | ||
| EGROUPEID | Event Group External ID | Text | Char | $ | 128 | $512 | ||
| EVENT | Event | Text | Char | $ | 128 | $512 | ||
| EVENTDEF | Event Definition | Text | Char | $ | 128 | $512 | ||
| EVENTEID | Event External ID | Text | Char | $ | 128 | $512 | ||
| EVENTDT | Event Date | Date | Num | : vdate. | DATE9 | 14 | 8 | |
| VISMETHOD | Visit Method | Text | Char | $ | 100 | $400 | This column is only visible if Visit Method is enabled. | |
| PLANNEDDT | Planned Date | Date | Num | : vdate. | DATE9 | 14 | 8 | |
| ESEQ | Event Group Sequence | Integer | Num | 14 | 8 | |||
| LASTMODDAT | Last modified date/time of the event date (UTC) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| CASEBDEF | Casebook Version | Integer | Num | $ | 14 | 8 | ||
| STATUS | Status of the event/visit | Text | Char | $ | 100 | $400 | This field is populated for log events in version 25R1. Data Models 1 & 2 will pull from the summary object. | |
| LASTREAS | Last change reason | Text | Char | $ | 255 | $1020 | ||
| WINSTAT | Scheduled Window Status | Text | Char | $ | 32 | $128 | ||
| DAYSOW | Days Outside Window | Integer | Num | 14 | 8 | |||
| EXPFORMS | Expected Number of Forms | Integer | Num | 14 | 8 | This field is populated for log events in version 25R1. | ||
| FORMEOD | Number of days past due when one or more forms is not yet complete | Integer | Num | 14 | 8 | |||
| EVFROZEN | Event Frozen | Boolean | Char | $ | 5 | $20 | Populated for Data Model 2 studies only. This field is populated for log events in version 25R1. | |
| EVFREEZEDT | Event Frozen Datetime | Date | Num | : vdate. | DATETIME22.3 | 14 | 8 | Populated for Data Model 2 studies only. This field is populated for log events in version 25R1. |
| EVLOCKED | Event Locked | Boolean | Char | $ | 5 | $20 | Populated for Data Model 2 studies only. This field is populated for log events in version 25R1. | |
| EVLOCKDT | Event Locked Datetime | Date | Num | : vdate. | DATETIME22.3 | 14 | 8 | Populated for Data Model 2 studies only. This field is populated for log events in version 25R1. |
| EVSIGNED | Event Signed | Boolean | Char | $ | 5 | $20 | Populated for Data Model 2 studies only. This field is populated for log events in version 25R1. | |
| EVSIGNDT | Event Signed Datetime | Date | Num | : vdate. | DATETIME22.3 | 14 | 8 | Populated for Data Model 2 studies only. This field is populated for log events in version 25R1. |
| GUID | Internal Vault ID of the event | Text | Char | $ | 100 | $400 | For HVO objects, the Length is 15, SAS Length is 60 | |
| LASTRUN | Last run of this data/listing | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 |
SYS_FORM
The SYS_FORM dataset lists all forms for the selected study, including forms Intentionally Left Blank as well as review statuses, freeze and lock statuses, and additional metrics.
List of SYS_FORM columns:
| Column Header | Label | Type | SAS Type | SAS Informat | SAS Format | Length | SAS Length | Notes |
|---|---|---|---|---|---|---|---|---|
| STUDYID | Study | Text | Char | $ | 128 | $512 | ||
| COUNTRY | Country | Text | Char | $ | 128 | $512 | ||
| SITENUM | Study Site Number | Text | Char | $ | 128 | $512 | ||
| SUBJID | Subject | Text | Char | $ | 128 | $512 | ||
| EGROUP | Event Group | Text | Char | $ | 128 | $512 | ||
| EGROUPDEF | Event Group Definition | Text | Char | $ | 128 | $512 | ||
| EGROUPEID | Event Group External ID | Text | Char | 128 | $512 | |||
| EVENT | Event | Text | Char | $ | 128 | $512 | ||
| EVENTDEF | Event Definition | Text | Char | $ | 128 | $512 | ||
| EVENTEID | Event External Id | Text | Char | $ | 128 | $512 | ||
| EVENTDT | Event date | Date | Num | : vdate. | DATE9. | 14 | 8 | |
| ESEQ | Event Group Sequence | Integer | Num | 14 | 8 | |||
| FORM | Form | Text | Char | $ | 128 | $512 | ||
| FORMDEF | Form Definition | Text | Char | $ | 128 | $512 | ||
| FORMEID | Form External ID | Text | Char | $ | 128 | $512 | ||
| FSEQ | Form Sequence | Integer | Num | 14 | 8 | |||
| STATUS | Form Status | Text | Char | $ | 100 | $400 | ||
| CREATEDT | Datetime form created | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| FIRSTSUBMITDT | Datetime form first submitted | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | This field will only be populated for new studies created after 23R3. |
| LASTSUBMITDT | Datetime form last submitted | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| NSUBMITS | Number of times form was submitted | Integer | Num | 14 | 8 | |||
| SDVOVRPLAN | SDV Override Plan | Text | Char | $ | 128 | $512 | Populated for Data Model 2 studies only. | |
| SDVREQ | SDV Required | Boolean | Char | $ | 5 | $20 | Not supported for SDV rollup V1 studies, will be blank | |
| SDVCOMP | SDV Complete | Boolean | Char | $ | 5 | $20 | Populated for SDV Rollup v2 studies only. | |
| SDVDT | Datetime considered SDV complete (UTC) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| FIRSTSDVDT | Datetime Form First SDV Complete | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| SDVLAST | Datetime of last SDV modification (UTC) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | Not supported for SDV rollup V1 studies, will be blank |
| TOSDV | Days from submit to SDV | Float | Num | 14 | 8 | |||
| DMROVRPLAN | DMR Override Plan | Text | Char | $ | 128 | $512 | Populated for Data Model 2 studies only. | |
| DMRREQ | DMR Required | Boolean | Char | $ | 5 | $20 | Not supported for SDV rollup V1 studies, will be blank | |
| DMRCOMP | DMR Complete | Boolean | Char | $ | 5 | $20 | Populated for SDV Rollup v2 studies only. | |
| DMRDT | Datetime considered DMR complete | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| FIRSTDMRDT | Datetime Form First DMR Complete | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| DMRLAST | Datetime of last DMR modification (UTC) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | Not supported for SDV rollup V1 studies, will be blank |
| TODMR | Days from submit to DMR | Float | Num | 14 | 8 | |||
| FROZEN | Form Frozen | Boolean | Char | $ | 5 | $20 | Populated for Data Model 2 studies only. | |
| FREEZEDT | Datetime frozen (UTC) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| TOFREEZE | Days from submit to freeze | Float | Num | 14 | 8 | |||
| LOCKED | Form Locked | Boolean | Char | $ | 5 | $20 | Populated for Data Model 2 studies only. | |
| LOCKDT | Datetime locked (UTC) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| TOLOCK | Days from submit to lock | Float | Num | 14 | 8 | |||
| SIGNED | Form Signed | Boolean | Char | $ | 5 | $20 | Populated for Data Model 2 studies only. | |
| SIGNDT | Datetime signed (UTC) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| LSTSGNDT | Last Signature Date | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| TOSIGN | Days from submit to sign | Float | Num | 14 | 8 | |||
| EDT_SUBM | Days from event date (of form) to submit date | Float | Num | 14 | 8 | |||
| ILB | Intentionally Left Blank | Boolean | Char | $ | 5 | $20 | ||
| ILBREAS | Reason for Intentionally Left Blank | Text | Char | $ | 255 | $1020 | ||
| GUID | Internal Vault ID of the form | Text | Char | $ | 100 | $400 | For HVO objects, the Length is 15, SAS Length is 60 | |
| LASTRUN | Last run of this data/listing | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 |
SYS_Q
The SYS_Q dataset lists all queries for the selected study, along with some query metrics. Only the first query message is listed in this dataset. The full list of query messages (first message, answers, etc.) is included in the SYS_QT dataset.
If you have the Show Queries in the User Language setting configured, queries will display in the user’s language, not the vault language.
List of SYS_Q columns:
| Column Header | Label | Type | SAS Type | SAS Informat | SAS Format | Length | SAS Length | Notes |
|---|---|---|---|---|---|---|---|---|
| STUDYID | Study | Text | Char | $ | 128 | $512 | ||
| COUNTRY | Country | Text | Char | $ | 128 | $512 | ||
| SITENUM | Study Site Number | Text | Char | $ | 128 | $512 | ||
| SUBJID | Subject | Text | Char | $ | 128 | $512 | ||
| EGROUP | Event Group | Text | Char | $ | 128 | $512 | ||
| EGROUPDEF | Event Group Definition | Text | Char | $ | 128 | $512 | ||
| EGROUPEID | Event Group External ID | Text | Char | 128 | 512 | |||
| EVENT | Event | Text | Char | $ | 128 | $512 | ||
| EVENTDEF | Event Definition | Text | Char | $ | 128 | $512 | ||
| EVENTEID | Event External Id | Text | Char | $ | 128 | $512 | ||
| ESEQ | Event Group Sequence | Integer | Num | 14 | 8 | |||
| FORM | Form | Text | Char | $ | 128 | $512 | ||
| FORMDEF | Form Definition | Text | Char | $ | 128 | $512 | ||
| FORMEID | Form External ID | Text | Char | $ | 128 | $512 | ||
| FSEQ | Form Sequence | Integer | Num | 14 | 8 | |||
| IGROUP | Item Group | Text | Char | $ | 500 | $2000 | ||
| IGROUPDEF | Item Group Definition | Text | Char | $ | 128 | $512 | ||
| IGROUPEID | Item Group External ID | Text | Char | $ | 128 | $512 | ||
| IGROUPDEF | Item Group Definition Name | Text | Char | $ | 128 | 512 | ||
| IGSEQ | Item group sequence | Integer | Num | 14 | 8 | |||
| ITEM | Item | Text | Char | $ | 500 | $2000 | ||
| ITEMDEF | Item Definition | Text | Char | $ | 128 | $512 | ||
| ITEMEID | Item External ID | Text | Char | 128 | $512 | |||
| QUERYID | Query ID | Text | Char | $ | 128 | $512 | ||
| STATUS | Query status | Text | Char | $ | 100 | $400 | Includes Open, Answered, and Closed queries. | |
| RESTRICTED | Query Restricted | Boolean | Char | 5 | $20 | Visible if "Include Restricted Data" is selected in SDE Version 23R1. | ||
| MANUAL | Manual query | Boolean | Char | $ | 5 | $20 | ||
| RULEID | Rule name | Text | Char | $ | 128 | $512 | ||
| TRIGID | Trigger name | Text | Char | $ | 128 | $512 | ||
| QTEXT | Initial query text/message | Text | Char | $ | 500 | $2000 | ||
| QTEXTBASE | Initial query text/message in the user’s language | Text | Char | $ | 500 | $2000 | Will be populated in SDE Version 24R3. May show as blank in some cases. | |
| QTEXTENG | Initial query text/message in English | Text | Char | $ | 500 | $2000 | ||
| OBSSOURCEVAL | Observed Source Value | Text | Char | $ | $25 | $100 | Will be populated for studies using Quick Queries. | |
| CREATED | Created date/time (UTC) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| CREATEDB | Created by | Text | Char | $ | 201 | $804 | ||
| LASTCLOSEDDT | Query Last Closed Date | Datetime | Num | :vdatetime. | DATETIME22.3 | 14 | 8 | |
| QUERYTEAM | Query team | Text | Char | $ | 50 | $200 | ||
| QTFRESP | Time to first response (days) | Float | Num | 14 | 8 | Only populated when there's a First Response Date and the query has a status of "Answered." | ||
| QAGE | Query Age (days) | Float | Num | 14 | 8 | |||
| QCHGDATA | Whether the data changed after query creation (field query attached) | Boolean | Char | $ | 5 | $20 | ||
| QOTOCL | Query open to close (days) | Float | Num | 14 | 8 | |||
| SRCTYPE | Source Type | Text | Char | $ | 30 | $120 | ||
| SRCSYS | Source System Name | Text | Char | $ | 100 | $400 | ||
| SRCUSER | Source User | Text | Char | $ | 100 | $400 | ||
| SRCID | Source ID | Text | Char | $ | 64 | $256 | ||
| GUID | Internal Vault ID of the query | Text | Char | $ | 100 | $400 | For HVO objects, the Length is 15, SAS Length is 60 | |
| LASTRUN | Last run of this data/listing | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 |
SYS_QT
The SYS_QT dataset lists all query messages for the selected study.
You can link the content of this file with the SYS_Q dataset using the QUERYID column. There can be multiple SYS_QT records matching one SYS_Q record (i.e. a query in SYS_Q can have multiple query messages in SYS_QT).
If you have the Show Queries in the User Language setting configured, queries will display in the user’s language, not the vault language.
List of SYS_QT columns:
| Column Header | Label | Type | SAS Type | SAS Informat | SAS Format | Length | SAS Length | Notes |
|---|---|---|---|---|---|---|---|---|
| STUDYID | Study | Text | Char | $ | 128 | $512 | ||
| COUNTRY | Country | Text | Char | $ | 128 | $512 | ||
| SITENUM | Study Site Number | Text | Char | $ | 128 | $512 | ||
| SUBJID | Subject | Text | Char | $ | 128 | $512 | ||
| QUERYID | Query ID | Text | Char | $ | 128 | $512 | ||
| STATUS | Query status | Text | Char | $ | 100 | $400 | Includes Open, Answered, Closed, and Reopened queries | |
| RESTRICTED | Query Restricted | Boolean | Char | 5 | $20 | Visible if "Include Restricted Data" is selected in SDE Version 23R1. | ||
| QTEXT | Initial query text/message | Text | Char | $ | 500 | $2000 | ||
| QTEXTBASE | Initial query text/message in the user’s language | Text | Char | $ | 500 | $2000 | Will be populated in SDE Version 24R3. May show as blank in some cases. | |
| QTEXTENG | Initial query text/message in English | Text | Char | $ | 500 | $2000 | Will be populated in SDE Version 24R3. May show as blank in some cases. | |
| TEXTDT | Text/Message Date/Time (UTC) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| TEXTBY | Text/Message By | Text | Char | $ | 201 | $1804 | ||
| QUERYTEAM | Query team | Text | Char | $ | 50 | $200 | ||
| QUICKACT | Quick Action | Boolean | Char | $ | 5 | $20 | Will be populated for studies using Quick Queries | |
| QUICKACTTYPE | Quick Action Type | Text | Char | $ | 100 | $400 | Will be populated for studies using Quick Queries | |
| SRCTYPE | Message Source Type | Text | Char | $ | 30 | $120 | ||
| SRCSYS | Message Source System Name | Text | Char | $ | 100 | $400 | ||
| SRCUSER | Message Source User | Text | Char | $ | 100 | $400 | ||
| SRCID | Message Source ID | Text | Char | $ | 64 | $256 | ||
| GUID | Internal Vault ID of the query message | Text | Char | $ | 100 | $400 | For HVO objects, the Length is 15, SAS Length is 60 | |
| LASTRUN | Last run of this data/listing | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 |
SYS_ILB
The SYS_ILB dataset lists all items that have been marked Intentionally Left Blank in the selected study.
List of SYS_ILB columns:
| Column Header | Label | Type | SAS Type | SAS Informat | SAS Format | Length | SAS Length | Notes |
|---|---|---|---|---|---|---|---|---|
| STUDYID | Study | Text | Char | $ | 128 | $512 | ||
| COUNTRY | Country | Text | Char | $ | 128 | $512 | ||
| SITENUM | Study Site Number | Text | Char | $ | 128 | $512 | ||
| SUBJID | Subject | Text | Char | $ | 128 | $512 | ||
| EGROUP | Event Group | Text | Char | $ | 128 | $512 | ||
| EGROUPDEF | Event Group Definition | Text | Char | $ | 128 | $512 | ||
| EGROUPEID | Event Group External ID | Text | Char | 128 | $512 | |||
| EVENT | Event | Text | Char | $ | 128 | $512 | ||
| EVENTDEF | Event Definition | Text | Char | $ | 128 | $512 | ||
| EVENTEID | Event External Id | Text | Char | $ | 128 | $512 | ||
| ESEQ | Event Group Sequence | Integer | Num | 14 | 8 | |||
| FORM | Form | Text | Char | $ | 128 | $512 | ||
| FORMDEF | Form Definition | Text | Char | $ | 128 | $512 | ||
| FORMEID | Form External ID | Text | Char | $ | 128 | $512 | ||
| FSEQ | Form Sequence | Integer | Num | 14 | 8 | |||
| IGROUP | Item Group | Text | Char | $ | 500 | $2000 | ||
| IGROUPDEF | Item Group Definition Name | Text | Char | 128 | 512 | |||
| IGROUPEID | Item Group External ID | Text | Char | 128 | 512 | |||
| IGSEQ | Item Group Sequence | Integer | Num | 14 | 8 | |||
| ITEM | Item | Text | Char | $ | 500 | $2000 | ||
| ITEMDEF | Item Definition | Text | Char | $ | 128 | $512 | ||
| ITEMEID | Item External ID | Text | Char | $ | 128 | $512 | ||
| LABANALYTENAME | Analyte Name | Text | Char | $ | 128 | $512 | Visible if Local Labs is enabled | |
| ILBREAS | Reason for intentionally left blank | Text | Char | $ | 255 | $1020 | ||
| GUID | Internal Vault ID of the item/field | Text | Char | $ | 100 | $400 | For HVO objects (item2__v), the Length is 15, SAS Length is 60 | |
| LASTRUN | Last run of this data/listing | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 |
SYS_LINKS
The SYS_LINKS dataset lists all forms that belong to a form link in the selected study. Forms that are linked together will share the same link ID, which will display in the GUID column. The form unique identifier (FGUID) can then be used to identify a specific form and access its data.
List of SYS_LINKS columns:
| Column Header | Label | Type | SAS Type | SAS Informat | SAS Format | Length | SAS Length | Description |
|---|---|---|---|---|---|---|---|---|
| STUDYID | Study | Text | Char | $ | 128 | $512 | ||
| COUNTRY | Country | Text | Char | $ | 128 | $512 | ||
| SITENUM | Study Site Number | Text | Char | $ | 128 | $512 | ||
| SUBJID | Subject | Text | Char | $ | 128 | $512 | ||
| EGROUP | Event Group | Text | Char | $ | 128 | $512 | ||
| EGROUPDEF | Event Group Definition | Text | Char | $ | 128 | $512 | ||
| EGROUPEID | Event Group External ID | Text | Char | 128 | $512 | |||
| EVENT | Event | Text | Char | $ | 128 | $512 | ||
| EVENTDEF | Event Definition | Text | Char | $ | 128 | $512 | ||
| EVENTEID | Event External Id | Text | Char | $ | 128 | $512 | ||
| ESEQ | Event Group Sequence | Integer | Num | 14 | 8 | |||
| FORM | Form | Text | Char | $ | 128 | $512 | ||
| FORMDEF | Form Definition | Text | Char | $ | 128 | $512 | ||
| FORMEID | Form external ID | Text | Char | $ | 128 | $512 | ||
| FSEQ | Form Sequence | Integer | Num | 14 | 8 | |||
| IGROUP | Item Group | Text | Char | $ | 500 | $2000 | ||
| IGROUPDEF | Item Group Definition | Text | Char | $ | 128 | $512 | ||
| IGROUPEID | Item Group External ID | Text | Char | $ | 128 | $512 | ||
| IGSEQ | Item Group Sequence | Integer | Num | 14 | 8 | |||
| ITEM | Item | Text | Char | $ | 500 | $2000 | Only visible if Form Linking is enabled. | |
| ITEMDEF | Item Definition | Text | Char | $ | 128 | $512 | Only visible if Form Linking is enabled. | |
| ITEMEID | Item External ID | Text | Char | 128 | $512 | Only visible if Form Linking is enabled. | ||
| FORMCREATEDDT | Form Created date/time (UTC) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| LINKCREATEDDT | Link Created Date/Time (UTC) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| CREATEDB | Created by | Text | Char | $ | 201 | $804 | ||
| FGUID | Internal Vault ID of the form | Text | Char | $ | 100 | $400 | For HVO objects, the Length is 15, SAS Length is 60 | |
| GUID | Internal Link ID | Text | Char | $ | 100 | $400 | For HVO objects, the Length is 15, SAS Length is 60 | |
| LASTRUN | Last run of this data/listing | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 |
SYS_ASM
The SYS_ASM dataset lists the following information about assessments, if assessments are configured for a Study.
| Column Header | Label | Type | SAS Type | SAS Informat | SAS Format | Length | SAS Length | Notes |
|---|---|---|---|---|---|---|---|---|
| STUDYID | Study | Text | Char | $ | 128 | $512 | ||
| COUNTRY | Country | Text | Char | $ | 128 | $512 | ||
| SITENUM | Study Site Number | Text | Char | $ | 128 | $512 | ||
| SUBJID | Subject | Text | Char | $ | 128 | $512 | ||
| EGROUP | Event Group | Text | Char | $ | 128 | $512 | ||
| EGROUPDEF | Event Group Definition | Text | Char | $ | 128 | $512 | ||
| EGROUPEID | Event Group External ID | Text | Char | $ | 128 | $512 | ||
| EVENT | Event | Text | Char | $ | 128 | $512 | ||
| EVENTDEF | Event Definition | Text | Char | $ | 128 | $512 | ||
| EVENTEID | Event External ID | Text | Char | $ | 128 | $512 | ||
| ESEQ | Event Group Sequence | Integer | Num | 14 | 8 | |||
| SOURCEF | Form | Text | Char | $ | 128 | $512 | ||
| SOURCEFDEF | Form Definition | Text | Char | $ | 128 | $512 | ||
| SOURCEFEID | Form External ID | Text | Char | $ | 128 | $512 | ||
| FSEQ | Form Sequence | Integer | Num | 14 | 8 | |||
| ASMNAME | Assessment Name | Text | Char | $ | 128 | $512 | ||
| ASMLABEL | Assessment Label | Text | Char | $ | 128 | $512 | ||
| ASMEID | Assessment External ID | Text | Char | $ | 128 | $512 | ||
| ASMTYPE | Assessment Type | Text | Char | $ | 128 | $512 | ||
| REASSESSMENT | Reassessment | Boolean | Char | $ | 5 | $20 | ||
| SOURCEF | Source Form | Text | Char | $ | 128 | $512 | ||
| SOURCEFDEF | Form Definition | Text | Char | $ | 128 | $512 | ||
| STATUS | Status of Assessment | Text | Char | $ | 100 | $400 | ||
| SOURCEFRESTRICTED | Source Form Restricted | Boolean | Char | $ | 5 | $20 | ||
| CREATEDT | Created Date/Time (UTC) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| CREATEDB | Created By | Text | Char | $ | 201 | $804 | ||
| COMPDT | Completed Date/Time (UTC) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| NSUBMITS | Submit Count | Integer | Num | 14 | 8 | |||
| LASTMODDT | Last Modified Date/Time (UTC) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| LASTMODB | Last Modified By | Text | Char | $ | 201 | $804 | ||
| GUID | Assessment Vault ID | Text | Char | $ | 100 | $400 | For HVO objects, the Length is 15, SAS Length is 60 | |
| FGUID | Internal Vault ID of the form | Text | Char | $ | 100 | $400 | For HVO objects, the Length is 15, SAS Length is 60 | |
| LASTRUN | Last run of this data/listing | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 |
SYS_ASMR
The SYS_ASMR dataset lists questions and answers for all assessments in a Study, if assessments are configured.
| Column Header | Label | Type | SAS Type | SAS Informat | SAS Format | Length | SAS Length | Notes |
|---|---|---|---|---|---|---|---|---|
| STUDYID | Study | Text | Char | $ | 128 | $512 | ||
| COUNTRY | Country | Text | Char | $ | 128 | $512 | ||
| SITENUM | Study Site Number | Text | Char | $ | 128 | $512 | ||
| SUBJID | Subject | Text | Char | $ | 128 | $512 | ||
| EGROUP | Event Group | Text | Char | $ | 128 | $512 | ||
| EGROUPDEF | Event Group Definition | Text | Char | $ | 128 | $512 | ||
| EGROUPEID | Event Group External ID | Text | Char | $ | 128 | $512 | ||
| EVENT | Event | Text | Char | $ | 128 | $512 | ||
| EVENTDEF | Event Definition | Text | Char | $ | 128 | $512 | ||
| EVENTEID | Event External ID | Text | Char | $ | 128 | $512 | ||
| ESEQ | Event Group Sequence | Integer | Num | 14 | 8 | |||
| SOURCEF | Form | Text | Char | $ | 128 | $512 | ||
| SOURCEFDEF | Form Definition | Text | Char | $ | 128 | $512 | ||
| SOURCEFEID | Form External ID | Text | Char | $ | 128 | $512 | ||
| FSEQ | Form Sequence | Integer | Num | 14 | 8 | |||
| QUESNUM | Question Number | Integer | Num | 14 | 8 | |||
| ASMNAME | Assessment Name | Text | Char | $ | 128 | $512 | ||
| ASMLABEL | Assessment Label | Text | Char | $ | 128 | $512 | ||
| ASMEID | Assessment External ID | Text | Char | $ | 128 | $512 | ||
| QUESTEXT | Question Text | Text | Char | $ | 128 | $512 | ||
| QUESTEXTDEF | Question Text Definition | Text | Char | $ | 128 | $512 | ||
| QUESEID | Question External ID | Text | Char | $ | 128 | $512 | ||
| QUESANS | Question Answer | Text | Char | $ | 1500 | $6000 | ||
| SOURCEFRESTRICTED | Source Form Restricted | Boolean | Char | $ | 5 | $20 | ||
| CREATEDT | Created Date/Time (UTC) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| CREATEDB | Created By | Text | Char | $ | 201 | $804 | ||
| LASTMODDT | Last Modified Date/Time (UTC) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| LASTMODB | Last Modified By | Text | Char | $ | 201 | $804 | ||
| AGUID | Assessment Vault ID | Text | Char | $ | 100 | $400 | For HVO objects, the Length is 15, SAS Length is 60 | |
| LASTRUN | Last run of this data/listing | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 |
SYS_PD
The SYS_PD dataset lists the following Protocol Deviations information, if Protocol Deviations is enabled.
| Column Header | Label | Type | SAS Type | SAS Informat | SAS Format | Length | SAS Length | Notes |
|---|---|---|---|---|---|---|---|---|
| STUDYID | Study | Text | Char | $ | 128 | $512 | ||
| COUNTRY | Country | Text | Char | $ | 128 | $512 | ||
| SITENUM | Study Site Number | Text | Char | $ | 128 | $512 | ||
| SUBJID | Subject | Text | Char | $ | 128 | $512 | ||
| PDID | Protocol Deviation Identifier | Text | Char | $ | 128 | $512 | ||
| PDSUM | Protocol Deviation Summary | Text | Char | $ | 250 | $1000 | ||
| PDDAT | Date of Deviation | Date | Num | : vdate. | DATE9. | 14 | 8 | |
| PDDATID | Date Identified | Date | Num | : vdate. | DATE9. | 14 | 8 | |
| PDCAT | Category | Text | Char | $ | 128 | $512 | ||
| PDCATLABEL | Category Label | Text | Char | $ | 128 | $512 | ||
| PDSUBCAT | Subcategory | Text | Char | $ | 128 | $512 | ||
| PDSUBCATLABEL | Subcategory Label | Text | Char | $ | 128 | $512 | ||
| PDSEV | Severity | Text | Char | $ | 128 | $512 | ||
| PDSEVLABEL | Severity Label | Text | Char | $ | 128 | $512 | ||
| PDDESC | Description | Text | Char | $ | 500 | $2000 | ||
| PDSTATUS | Protocol Deviation Status | Text | Char | $ | 100 | $400 | ||
| PDRES | Protocol Deviation Resolution | Text | Char | $ | 500 | $2000 | ||
| PDRULE | Protocol Deviation Rule | Text | Char | $ | 128 | $512 | ||
| RESTRICTED | Protocol Deviation Restricted | Boolean | Char | 5 | $20 | |||
| EGROUP | Event Group | Text | Char | $ | 128 | $512 | ||
| EGROUPDEF | Event Group Definition | Text | Char | $ | 128 | $512 | ||
| EGROUPEID | Event Group External ID | Text | Char | 128 | $512 | |||
| EVENT | Event | Text | Char | $ | 128 | $512 | ||
| EVENTDEF | Event Definition | Text | Char | $ | 128 | $512 | ||
| EVENTEID | Event External ID | Text | Char | $ | 128 | $512 | ||
| ESEQ | Event Group Sequence | Integer | Num | 14 | 8 | |||
| FORM | Form | Text | Char | $ | 128 | $512 | ||
| FORMDEF | Form Definition | Text | Char | $ | 128 | $512 | ||
| FORMEID | Form External ID | Text | Char | $ | 128 | $512 | ||
| FSEQ | Form Sequence | Integer | Num | 14 | 8 | |||
| IGROUP | Item Group | Text | Char | $ | 500 | $2000 | ||
| IGROUPDEF | Item Group | Text | Char | $ | 128 | $512 | ||
| IGROUPEID | Item Group External ID | Text | Char | $ | 128 | $512 | ||
| IGSEQ | Item Group Sequence | Integer | Num | 14 | 8 | |||
| ITEM | Item | Text | Char | $ | 500 | $2000 | ||
| ITEMDEF | Item Definition | Text | Char | $ | 128 | $512 | ||
| ITEMEID | Item External ID | Text | Char | 128 | $512 | |||
| CREATEDT | Created Date/Time (UTC) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| CREATEDB | Created By | Text | Char | $ | 201 | $804 | ||
| LASTMODDT | Last Modified Date/Time (UTC) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| LASTMODB | Last Modified By | Text | Char | $ | 201 | $804 | ||
| USERMODDT | User Modified Date | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| USERMODB | User Modified By | Text | Char | $ | 201 | $804 | ||
| LASTREAS | Last Change Reason | Text | Char | $ | 255 | $1020 | ||
| INACBYSYS | Inactivated by System | Boolean | Char | $ | 5 | $20 | ||
| LASTINACDT | Last Inactivated Date | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| FGUID | Internal Vault ID of Form | Text | Char | $ | 100 | $400 | For HVO objects, the Length is 15, SAS Length is 60 | |
| PDGUID | Internal Vault ID of Protocol Deviation | Text | Char | $ | 15 | $60 | For HVO objects, the Length is 15, SAS Length is 60 | |
| LASTRUN | Last run of this data/listing | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 |
SYS_RAND
The SYS_RAND dataset lists the following Randomization information, if Randomization is enabled.
| Column Header | Label | Type | SAS Type | SAS Informat | SAS Format | Length | SAS Length | Notes |
|---|---|---|---|---|---|---|---|---|
| STUDYID | Study | Text | Char | $ | 128 | $512 | ||
| COUNTRY | Country | Text | Char | $ | 128 | $512 | ||
| SITE | Study Site Number | Text | Char | $ | 128 | $512 | ||
| SUBJID | Subject | Text | Char | $ | 128 | $512 | ||
| RANDSTATUS | Randomization Status | Text | Char | $ | 100 | $400 | ||
| RANDID | Randomization ID | Text | Char | $ | 128 | $512 | ||
| TREATMENTNAME* | Treatment Name | Text | Char | $ | 128 | $512 | ||
| TREATMENTLABEL* | Treatment Label | Text | Char | $ | 1500 | $6000 | ||
| TREATMENTARM* | Treatment Arm | Text | Char | $ | 128 | $512 | Visible if "Include Randomization Treatment" is selected in SDE Version 22R1 or later | |
| RANDSTRATA | Strata Group | Text | Char | $ | 128 | $512 | ||
| RANDBY | Randomized By | Text | Char | $ | 201 | $804 | ||
| RANDDAT | Randomized Date | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| RANDFILE | Randomization File Label | Text | Char | $ | 128 | $512 | ||
| RANDFILEDAT | Randomization File Upload Date | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| LASTRUN | Last run of this data/listing | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 |
*These columns are only available for unmasked studies with Randomization enabled and users with the View Unmasked Data and View Randomization Enrollment permissions. To include these columns, check the Include Randomization Treatment box in the New Job dialog. Note that you can’t download SDE files from users with the View Randomization Enrollment permission if the Include Randomization Treatment checkbox is not visible to you in the SDE job dialog.
SYS_LABRANGES
The SYS_LABRANGES dataset lists the following Labs information, if Labs is enabled.
| Column Header | Label | Type | SAS Type | SAS Informat | SAS Format | Length | SAS Length |
|---|---|---|---|---|---|---|---|
| LABID | Lab ID | Text | Char | $ | 128 | $512 | |
| LABLOC | Lab Location | Text | Char | $ | 128 | $512 | |
| LABANALYTENAME | Analyte Name | Text | Char | $ | 128 | $512 | |
| LABANALYTELABEL | Analyte Label | Text | Char | $ | 128 | $512 | |
| LABREFRSTATUS | Lab Reference Range Status | Text | Char | $ | 100 | $400 | |
| LABTESTCODE | Test Code | Text | Char | $ | 128 | $512 | |
| LABSEX | Sex | Text | Char | $ | 128 | $512 | |
| LABLOWERAGE | Lower Age | Integer | Num | 14 | 8 | ||
| LABLOWERAGEUNIT | Lower Age Unit | Text | Char | $ | 128 | $512 | |
| LABUPPERAGE | Upper Age | Integer | Num | 14 | 8 | ||
| LABUPPERAGEUNIT | Upper Age Unit | Text | Char | $ | 128 | $512 | |
| LBFAST | Fasting Status | Text | Char | $ | 128 | $512 | |
| LABFEMALECYCLE | Female Cycle | Text | Char | $ | 128 | $512 | |
| LABLNORMAL | Lower Normal | Float | Num | 14 | 8 | ||
| LABUNORMAL | Upper Normal | Float | Num | 14 | 8 | ||
| LABMEASUNIT | Measurement Unit | Text | Char | $ | 128 | $512 | |
| LABMEASUNITLABEL | Measurement Unit Label | Text | Char | $ | 128 | $512 | |
| LABCODENORM | Codelist Normal | Text | Char | $ | 128 | $512 | |
| LABTEXTNORM | Text Normal | Text | Char | $ | 3000 | $12000 | |
| LABMODIFIER | Lab Modifier | Boolean | Char | $ | 5 | $20 | |
| LABFROMDT | Effective From Date | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 |
| LABTODT | Effective To Date | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 |
| LABDESCR | Description | Text | Char | $ | 500 | $2000 | |
| LABSPECTYPE | Specimen Type | Text | Char | $ | 128 | $512 | |
| LABTESTMET | Testing Method | Text | Char | $ | 128 | $512 | |
| CREATB | Created By | Text | Char | $ | 201 | $804 | |
| CREATEDT | Created Date/Time (UTC) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 |
| LASTMODB | Last Modified By | Text | Char | $ | 201 | $804 | |
| LASTMODDT | Last Modified Date/Time (UTC) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 |
| LASTRUN | Last run of this data/listing | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 |
SYS_LABLOC
The SYS_LABLOC dataset lists the following Labs information, if Labs is enabled.
| Column Header | Label | Type | SAS Type | SAS Informat | SAS Format | Length | SAS Length | Notes |
|---|---|---|---|---|---|---|---|---|
| LABID | Lab ID | Text | Char | $ | 128 | $512 | ||
| LABLOC | Lab Location | Text | Char | $ | 128 | $512 | ||
| LABLOCSTATUS | Lab Location Status | Text | Char | $ | 100 | $400 | ||
| LABAPPROVED | Approved Lab Location | Text | Char | $ | 128 | $512 | ||
| LABADDRESS | Lab Address | Text | Char | $ | 500 | $2000 | ||
| LABCOUNTRY | Lab Country | Text | Char | $ | 128 | $512 | ||
| LABTIMEZONE | Lab Timezone | Text | Char | $ | 100 | $400 | ||
| LABCONTACT | Lab Contact Name | Text | Char | $ | 128 | $512 | ||
| LABCONTITLE | Lab Contact Title | Text | Char | $ | 128 | $512 | ||
| LABTEL | Lab Telephone | Text | Char | $ | 40 | $160 | ||
| LABFAX | Lab Fax | Text | Char | $ | 40 | $160 | ||
| LABPRIMEMAIL | Lab Primary Email | Text | Char | $ | 80 | $320 | ||
| LABSECMAIL | Lab Secondary Email | Text | Char | $ | 80 | $320 | ||
| LABSTATUS | Lab Status | Text | Char | $ | 100 | $400 | ||
| CREATEDB | Created By | Text | Char | $ | 201 | $804 | ||
| CREATEDT | Created Date/Time (UTC) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| LASTMODB | Last Modified By | Text | Char | $ | 201 | $804 | ||
| LASTMODDT | Last Modified Date/Time (UTC) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| GUID | Internal Vault ID | Text | Char | $ | 100 | $400 | For HVO objects, the Length is 15, SAS Length is 60 | |
| LASTRUN | Last run of this data/listing | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 |
SYS_ANALYTES
The SYS_ANALYTES dataset lists the following Labs analyte information, if Labs is enabled.
| Column Header | Label | Type | SAS Type | SAS Informat | SAS Format | Length | SAS Length | Notes |
|---|---|---|---|---|---|---|---|---|
| LABANALYTENAME | Analyte Name | Text | Char | $ | 128 | $512 | ||
| LABANALYTELABEL | Analyte Label | Text | Char | $ | 128 | $512 | ||
| LABDESCR | Description | Text | Char | $ | 500 | $2000 | ||
| LABDATATYPE | Data Type | Text | Char | $ | 100 | $400 | ||
| LABMEASUNIT | Measurement Unit | Text | Char | $ | 128 | $512 | ||
| LABCODELIST | Codelist | Text | Char | $ | 128 | $512 | ||
| LABMODIFIER | Lab Modifier | Boolean | Char | $ | 5 | $20 | ||
| LABSPECTYPE | Specimen Type | Text | Char | $ | 128 | $512 | ||
| LABLEN | Length | Integer | Num | 14 | 8 | |||
| LABPREC | Precision | Integer | Num | 14 | 8 | |||
| LABSYSMANAGED | System Managed | Boolean | Char | $ | 5 | $20 | ||
| LABTESTMET | Testing Method | Text | Char | $ | 128 | $512 | ||
| LABLOINC | LOINC Code | Text | Char | $ | 128 | $512 | ||
| LABSDTMNM | SDTM Name | Text | Char | $ | 255 | $1020 | ||
| CREATEDB | Created By | Text | Char | $ | 201 | $804 | ||
| CREATEDT | Created Date/Time (UTC) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| LASTMODB | Last Modified By | Text | Char | $ | 201 | $804 | ||
| LASTMODDT | Last Modified Date/Time (UTC) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| GUID | Internal Vault ID | Text | Char | $ | 100 | $400 | For HVO objects, the Length is 15, SAS Length is 60 | |
| LASTRUN | Last run of this data/listing | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 |
SYS_SAFC
The SYS_SAFC dataset lists the following Safety Case information for users with studies configured with a Safety Configuration.
| Column Header | Label | Type | SAS Type | SAS Informat | SAS Format | Length | SAS Length | Notes |
|---|---|---|---|---|---|---|---|---|
| STUDYID | Study | Text | Char | $ | 128 | $512 | ||
| COUNTRY | Study Country | Text | Char | $ | 128 | $512 | ||
| SITENUM | Study Site Number | Text | Char | $ | 128 | $512 | ||
| SUBJID | Subject | Text | Char | $ | 128 | $512 | ||
| CASEID | CDMS Case Identifier | Text | Char | $ | 128 | $512 | ||
| CASESTATUS | Current Case Status | Text | Char | $ | 100 | $400 | ||
| EVENTS | Events in Case | Text | Char | $ | 1500 | $60000 | ||
| WWUID | CDMS Safety Case Unique ID (WWUID) | Text | Char | $ | 100 | $400 | ||
| SAFUID | Safety System Case Unique ID | Text | Char | $ | 100 | $400 | ||
| FSEQ | Form Sequence of Primary Event | Integer | Num | 14 | 8 | |||
| REPDT | Date First Received by Source (UTC) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| MRECDT | Date Most Recent Info in Report (UTC) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| REPFIRSTNAME | First Reporter First Name | Text | Char | $ | 60 | $240 | ||
| REPLASTNAME | First Reporter Last Name | Text | Char | $ | 60 | $240 | ||
| REPCOUNTRY | First Reporter Country (ISO) | Text | Char | $ | 2 | $8 | ||
| LASTSFNAME | Last Send File Name | Text | Char | $ | 250 | $1000 | ||
| LASTMSGDT | Last Message Send to Safety (UTC) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| LASTACKFNAME | Last ACK File Name | Text | Char | $ | 250 | $1000 | ||
| FLLWUPINSP | Follow-up Inspection to Occur | Boolean | Char | $ | 5 | $20 | ||
| ADDFLLWUP | Additional Follow-up Pending | Boolean | Char | $ | 5 | $20 | ||
| CREATEDT | Created Date/Time (UTC) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| LASTMODDT | Last Modified Date/Time (UTC) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| GUID | Internal Vault ID of the Case | Text | Char | $ | 100 | $400 | For HVO objects, the Length is 15, SAS Length is 60 | |
| LASTRUN | Last run of this data/listing | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 |
SYS_SAFM
The SYS_SAFM dataset lists the following Safety Message information for users with studies configured with a Safety Configuration.
| Column Header | Label | Type | SAS Type | SAS Informat | SAS Format | Length | SAS Length | Notes |
|---|---|---|---|---|---|---|---|---|
| STUDYID | Study | Text | Char | $ | 128 | $512 | ||
| COUNTRY | Study Country | Text | Char | $ | 128 | $512 | ||
| SITENUM | Study Site Number | Text | Char | $ | 128 | $512 | ||
| SUBJID | Subject | Text | Char | $ | 128 | $512 | ||
| MSGID | CDMS Message Identifier | Text | Char | $ | 128 | $512 | ||
| CASEID | CDMS Case Identifier | Text | Char | $ | 128 | $512 | ||
| CASESTATUS | Current Case Status | Text | Char | $ | 100 | $400 | ||
| MSGSTATUS | Current Message Status | Text | Char | $ | 100 | $400 | ||
| MSGTYPE | Message Type | Text | Char | $ | 100 | $400 | ||
| SFNAME | Send File Name | Text | Char | $ | 250 | $1000 | ||
| MRECDT | Date Most Recent Info in Report (UTC) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| BATCHNUM | Message ID/Batch Number | Integer | Num | 14 | 8 | |||
| TRANSDT | Date of Transmission (UTC) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| TRANSATT | Transmission Attempts | Integer | Num | 14 | 8 | |||
| AS2MSGID | AS2 Message ID | Text | Char | $ | 100 | $400 | ||
| MDNDT | MDN Date Received (UTC) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| ACKRECDT | ACK Date Received (UTC) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| ACKFNAME | ACK File Name Received | Text | Char | $ | 250 | $1000 | ||
| ACKERROR | ACK Error Message | Text | Char | $ | 250 | $1000 | ||
| NULLIFYREASON | Nullify Reason | Text | Char | $ | 1500 | $6000 | ||
| CREATEDT | Created Date/Time (UTC) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| LASTMODDT | Last Modified Date/Time (UTC) | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| GUID | Internal Vault ID of the Message | Text | Char | $ | 100 | $400 | For HVO objects, the Length is 15, SAS Length is 60 | |
| LASTRUN | Last run of this data/listing | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 |
Form, Event, & Query Status
Below are statuses and their descriptions for forms, events, and queries.
Form Status
| Status | Description |
|---|---|
| Blank | The Form has not been submitted and has no queries, no autosaves, and no fields left blank. |
| In Progress | The Form has either not been submitted since the last data change or there are tasks that have not been completed on the Form. |
| Submitted | The Form has been submitted and there are no open tasks. |
| In Progress Post-Submit | During a retrospective casebook amendment, there is an option to set the form status to In Progress for any modified forms. |
Event Status
| Status | Description |
|---|---|
| Planned | The Event has no Actual Start Date or is scheduled in the future. |
| Blank | The Event has an Actual Start Date, but all forms are in the Planned status. |
| In Progress | The Event has an Actual Start Date and at least one form is not Submitted or has at least one task. This status can also indicate that at least one but not all Forms in the Event are frozen or locked. |
| Submitted | All Forms in the Event are submitted and no forms have a Planned or Blank status. |
| Did Not Occur | The Event did not occur during study conduct. |
Query Status
| Status | Description |
|---|---|
| Open | "Open" indicates that a query has not been answered. |
| Answered | "Answered" indicates that a query has been addressed. For example, the site has responded with a reason for the queried value. |
| Closed | "Closed" indicates that a query requires no further action or discussion. A CRA or Data Manager can reopen a closed query if needed. |
SAS Format
If you select SAS as the Export File Format when scheduling your Study Data Extract job, the generated ZIP file contains two additional folders:
- SAS: Contains sas7bdat files for each dataset
- XPT: Contains XPT files for each dataset
Key columns use fixed conversions to either num or char SAS types, whereas clinical data columns are converted to a specific SAS type based on the item data type in EDC.
SAS validations and formatting:
- Column names are limited to 32 characters. The system will truncate as necessary to append special suffixes like _TRANSU or _D.
- SAS labels are limited to 255 characters. Labels that exceed 250 characters are truncated and have an ellipsis (…) added to the label.
- ZIP file names are limited to 200 characters.
- If a dataset starts with a number, it will be prefixed with an underscore (_).
- If a clinical dataset has the same name as one of the system datasets, it will be appended with “_X” (X being a number that can be incremented).
- If a column header starts with a number, it will be prefixed with an underscore (_).
- If a value is entered with carriage returns (new line), these characters are replaced with spaces.
- If the same column header is present multiple times (same item used in multiple item groups in the same form), the system appends “_X” (X being a number that can be incremented) to the column header. For column headers that already have a suffix (e.g. _TRANS), the “_X” suffix will be appended after the special suffix is added (_TRANS_2).
- Double byte numbers will be replaced by their single byte equivalent
- SDE Versions 22R3 & later: SAS lengths in the SDE display as four (4) times the defined length in Studio due to UTF-8 encoding, where a character may take up to four bytes of space. Because of this, we want to ensure that data using double byte characters doesn’t get truncated in SAS.
You will see an additional column in your Study Definitions file for the SAS column that will show any column header manipulations that were made due to SAS format conventions.
To view column names (instead of SAS labels) in the SAS viewer, right click and select Display Labels.
Note that SDE versions 22R1 and later will automatically compress SAS zip files.
We use SAS version v9.4. SAS behavior will dynamically set the XPT version based on the input of the data, which can either be V5 (V5 transport), V8 (V8 extended transport), or V9 (V9 extended transport).
Unknown Date and Datetime Values
The following applies to unknown date and datetime values in the SDE:
- SAS data type and format will be represented as a character.
- For most columns in SDE versions 22R3 and later, the length will be 64 and the SAS length will be 256.
- Note that the Effective To Date and Effective From Date columns in the SYS_LABRANGES dataset, once configured, will have the length of 64 and SAS length of 256 for all SDE versions.
- If the datetime item is split into separate date and time parts, those date and time parts will also be represented as a character.
CSV Format
The following naming conventions apply to extracts that are exported as a CSV file as of SDE Version 23R1 and later:
- Column names are limited to 32 characters. The system will truncate as necessary to append special suffixes like _TRANSU or _D.
- CSV labels are limited to 255 characters. Labels that exceed 250 characters are truncated and have an ellipsis (…) added to the label.
- ZIP file names are limited to 200 characters.
- If a dataset starts with a number, it will be prefixed with an underscore (_).
- If a clinical dataset has the same name as one of the system datasets, it will be appended with “_X” (X being a number that can be incremented).
- If a column header starts with a number, it will be prefixed with an underscore (_).
- If a value is entered with carriage returns (new line), these characters are replaced with spaces.
- If the same column header is present multiple times (same item used in multiple item groups in the same form), the system appends “_X” (X being a number that can be incremented) to the column header. For column headers that already have a suffix (e.g. _TRANS), the “_X” suffix will be appended after the special suffix is added (_TRANS_2).
- Double byte numbers will be replaced by their single byte equivalent
FTP Connection
To configure an FTP connection, see Connecting to an FTP Server from EDC Tools.
Custom Objects
You can include custom objects in your exports by adding records to the Study Data Extract Custom Object Config object in Business Administration where you must specify the Name of the record, custom object name (objectname__c), and the Study that you’re going to run the SDE for.
Once you’ve configured a custom object and run the SDE job, you will receive a file named after your custom object label (CUSTOM_), which will have records for each custom object you configured.
The order of the columns in the custom object file follows the Page Layout configuration, with the exception of the GUID of the object always appearing at the beginning of the file.
Duplicate column names are appended with an _2, _3, etc. to prevent column name conflicts.
Note that custom object records must be unique within a study.
List of Custom Objects columns:
| Column Header | Label | Type | SAS Type | SAS Informat | SAS Format | Length | SAS Length | Notes |
|---|---|---|---|---|---|---|---|---|
| GUID | ID | Text | Char | $ | 100 | $400 | ||
| NAME | Name | Text | Char | $ | 128 | $512 | ||
| STATUS | Status | Text | Char | $ | 100 | $400 | ||
| CREATEDB | Created By | Text | Char | $ | 201 | $804 | ||
| CREATED | Created Date | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| MODIFIEDB | Modified By | Text | Char | $ | 201 | $804 | ||
| DLASTMOD | Last Modified Date | Datetime | Num | : vdatetime. | DATETIME22.3 | 14 | 8 | |
| OBJECT_TYPE | Object Type | Text | Char | $ | 40 | $160 | Visible if Object Types are enabled for Custom Objects |
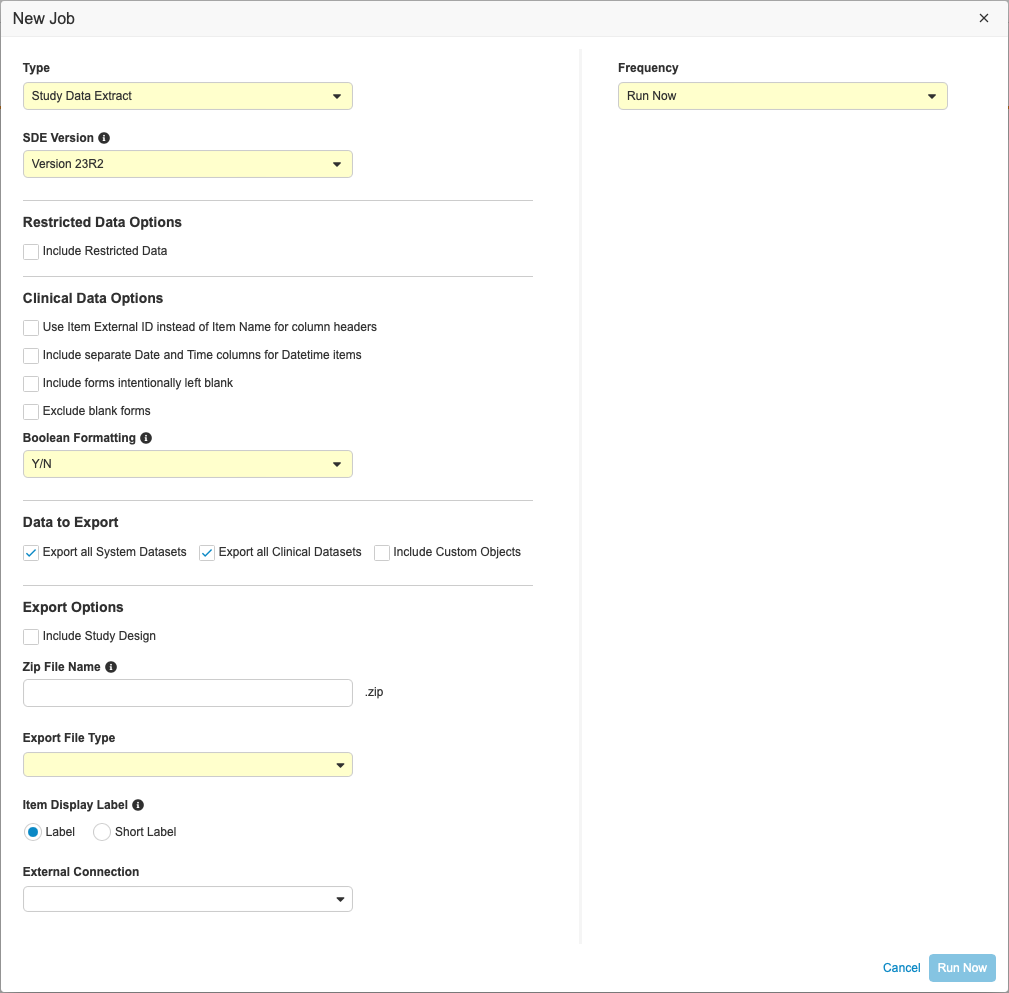
Running the Study Data Extract Job
To run the Study Data Extract job:
- Navigate to your Study in Tools > EDC Tools.
- Click Jobs.
- Click + New Job.
- Optional: Select Include Restricted Data to include data from restricted Forms in your extract.
- Optional: Select Include Randomization Treatment to include Randomization treatment information in the SYS_RAND dataset.
- Select which version of the SDE you’d like to run. Each job version determines which files are included in the extract and which version of the CSVs must be used. Note that the default version selected will be the latest version that is supported in the current release. See SDE Versioning for details.
- Optional: Make your selections for Clinical Data Options to determine how Vault handles clinical data formatting.
- Select a Boolean Formatting option. Note that boolean formatting applies to Clinical and System Datasets. See below for more information about boolean formatting options.
-
Optional: Select which datasets to export. All System Datasets and Clinical Datasets will be selected by default. To specify which datasets to export, uncheck the Export All checkbox.
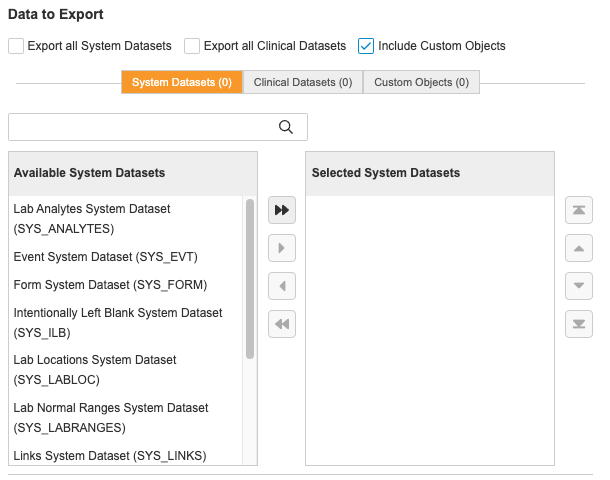
- Optional: Enter a ZIP File Name. This name is the filename that Vault will use for the generated ZIP file.
- Select one or more Export File Type.
- Choose your Item Display Label. The Item Display Label determines whether the label in the study definitions file and the SAS label will use the Item Label or Item Short Label as defined in Studio.
- Optional (only 22R3 Version): Select Include Study Design to include a Definitions folder containing study design information in your extract. See below for more information on the Include Study Design option.
- Optional: Select an External Connection to receive the output file. Otherwise, Vault will send you a link to download the output when the job finishes.
- Optional: Configure a Frequency to run the job on a recurring basis. Learn more about scheduled jobs.
- Click Run Now.
Boolean Formatting Options
You can choose from the following boolean formatting options when running the Study Data Extract job:
- Y/N
- Y/N/empty
- T/F
- T/F/empty
- 1/0
- 1/empty
- 1/0/empty
- true/false
- true/false/empty
- Yes/No
- Yes/No/empty
Options that contain “empty” will display a blank value if checkbox is unchecked. Values may also be blank if a field is not required.
Include Study Design (Version 22R3 and later)
The following files will be included in the Definitions folder when the Include Study Design option is checked:
- Export Options: Includes all export options saved in the job record
- Casebook Definitions: Includes all casebook versions
- Casebook Schedule: Includes all casebook versions
- Casebook Definition Summary: Includes information about the relationships between the Event Group, Event, Form, Item Group, and Items
- Event Definitions: Includes information about Events and Event Groups
- Event Group Repeat Overrides: Includes information about repeating Event Groups, if applicable. A record will appear in the Definitions folder due to system-generated defaults or if any of the Event Group Repeat Override Labels are modified.
- Form Definitions: Includes information about Forms
- Item Definitions: Includes information about Items and Item Groups
- Codelists: Includes Codelist information
- Unit Codelists: Includes Unit Codelist information
- Protocol Deviations: Includes information about Protocol Deviations, if applicable
- Protocol Deviations Relationships: Includes information about Protocol Deviations relationships, if applicable
- Medical Coding Items: Shows all medical coding items configured for a coding form, if applicable
- Medical Coding Related Items: Shows optionally configured related items on a coding form, if applicable
- Medical Coding Copy Indication from Links: Shows Form Links configured for Indication-related Item Types, if applicable
- Study Definitions: Includes information about the applicable study
The contents of the Casebook Schedule, Casebook Definition Summary, Item Definitions, and Form Definitions files will appear in the order configured in Studio.
Send Data to CDB (Version 23R2 and later)
In the 23R2 SDE version, users with CDB enabled can send SDE datasets to CDB, where the datasets will be handled like third-party data. The Study Label is used in the SDE, while the Study Name is used in CDB. Both values must be the same for the data to transfer successfully to CDB.
You can run two (2) Send to CDB Study Data Extract jobs outside regular SDE job governance limits.
To send SDE data to CDB:
- Start a new Study Data Extract job in EDC Tools > Job Schedule.
- Choose 23R2 under SDE Version.
-
Select the Send to CDB Workbench checkbox under the Send Data to CDB subsection and select a Manifest file. See example file below.
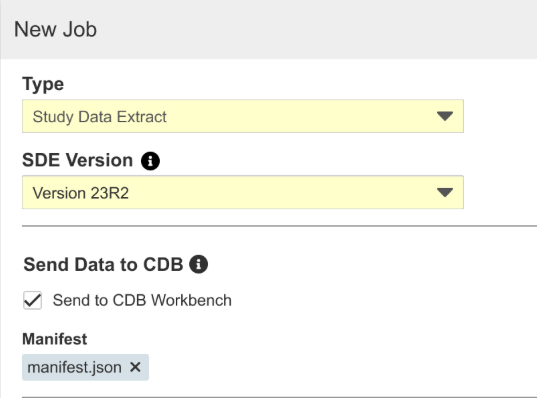
- Follow steps 5-17 in the Running the Study Data Extract section above. Note that you must select specific datasets to export (you can’t select the Export All Clinical Datasets option – we recommend not exporting clinical datasets) and you can only export your file as a CSV when sending to CDB.
Example Manifest File
See below for an example Manifest file. Note that this is an example file, so the custom object file name will be your custom object label.
Restricted data: If your file contains restricted data, you must mark any restricted data as blinded in your Manifest file.
{
"study": "Deetoza_DEV1",
"source": "SDE Data",
"blinded": false,
"data": [
{
"filename": "SYS_ASM.csv",
"study": "STUDYID",
"site": "SITENUM",
"subject": "SUBJID",
"rowid": {
"groupid": ["GUID"]
},
"event": {
"default": "baseline_visit"
},
"items": {
"COUNTRY": {
"type": "text"
},
"ASMNAME": {
"type": "text"
},
"ASMLABEL": {
"type": "text"
},
"ASMEID": {
"type": "text"
},
"ASMTYPE": {
"type": "text"
},
"REASSESSMENT": {
"type": "text"
},
"SOURCEF": {
"type": "text"
},
"SOURCEFDEF": {
"type": "text"
},
"STATUS": {
"type": "text"
},
"CREATEDT": {
"type": "datetime",
"format": "yyyy-MM-dd'T'HH:mmZ"
},
"CREATEDB": {
"type": "text"
},
"COMPDT": {
"type": "datetime",
"format": "yyyy-MM-dd'T'HH:mmZ"
},
"NSUBMITS": {
"type": "integer"
},
"LASTMODDT": {
"type": "datetime",
"format": "yyyy-MM-dd'T'HH:mmZ"
},
"LASTMODB": {
"type": "text"
},
"GUID": {
"type": "text"
},
"FGUID": {
"type": "text"
},
"LASTRUN": {
"type": "datetime",
"format": "yyyy-MM-dd'T'HH:mmZ"
}
}
},
{
"filename": "SYS_ASMR.csv",
"study": "STUDYID",
"site": "SITENUM",
"subject": "SUBJID",
"rowid": {
"groupid": ["QUESTEXTDEF", "QUESANS", "AGUID"]
},
"event": {
"default": "baseline_visit"
},
"items": {
"ASMNAME": {
"type": "text"
},
"ASMLABEL": {
"type": "text"
},
"ASMEID": {
"type": "text"
},
"QUESTEXT": {
"type": "text"
},
"QUESTEXTDEF": {
"type": "text"
},
"QUESANS": {
"type": "text"
},
"CREATEDT": {
"type": "datetime",
"format": "yyyy-MM-dd'T'HH:mmZ"
},
"CREATEDB": {
"type": "text"
},
"LASTMODDT": {
"type": "datetime",
"format": "yyyy-MM-dd'T'HH:mmZ"
},
"LASTMODB": {
"type": "text"
},
"AGUID": {
"type": "text"
},
"LASTRUN": {
"type": "datetime",
"format": "yyyy-MM-dd'T'HH:mmZ"
}
}
},
{
"filename": "CUSTOM_TEST_CUSTOM_OBJECT.csv",
"study": "STUDY",
"site": "SITE",
"subject": "SUBJECT",
"event": {
"default": "baseline_visit"
},
"rowid": {
"groupid": ["GUID"]
},
"items": {
"GUID": {
"type": "text"
},
"NAME": {
"type": "text"
},
"STATUS": {
"type": "text"
},
"CREATEDB": {
"type": "text"
},
"CREATED": {
"type": "datetime",
"format": "yyyy-MM-dd'T'HH:mm:ssZ"
},
"MODIFIEDB": {
"type": "text"
},
"DLASTMOD": {
"type": "datetime",
"format": "yyyy-MM-dd'T'HH:mm:ssZ"
},
"STUDY_COUNTRY": {
"type": "text"
}
}
}
]
}
Refer to Importing 3rd Party Data for more information about manifest files in CDB.
Troubleshooting
If extract jobs such as the Study Data Extract, Study Listings, or Detail PDF fail after removing or moving data from the study schedule in your DEV or TST environments, try deleting the casebook data before retrying the jobs. Deleting data such as Event Groups, Forms, Item Groups, and Items from the study schedule while it is still in the design process is not supported by EDC and will cause exports to fail.
To avoid extract jobs failing, keep an eye on the following changes that may cause extract job failure:
Changes in the Study Schedule:
- Removal of Event Groups, Events, Forms, Item groups, and Items
Changes in Business Admin
- Modification of object records relating to Events, Forms, Item Groups, and Items