Assessments Administration
Veeva EDC’s Medical Assessments feature supports the supplemental assessment and review of relevant casebook data. Selected subject casebook data is presented in the Assessments tab for medical review to inform determinations about that data. This feature can be used to support decision-making about trial data, including decisions regarding compliance, trial eligibility or continuation, and device deficiency. The Medical Assessments feature can also be used to complement an external adjudication process or a safety system such as Veeva Safety for additional high-level review. Assessment decisions and rationales are stored separately from the subject’s casebook data.
Medical monitors and other users in similar roles can perform medical assessments from within Veeva EDC. Before a user can perform a medical assessment, you must first assign a Study Role to that Assessment from EDC Tools > Assessments.
Prerequisites
A Study Designer can create an Assessment Definition from Studio > Assessments.
Users with the CDMS Lead Data Manager study role can perform the actions described above by default. If your vault uses custom Study Roles, your role must provide the following permissions:
| Type | Permission Label | Controls |
|---|---|---|
| Standard Tab | EDC Tools Tab | Ability to access the EDC Tools tab |
| Functional Permission | Manage Assessments | Ability to assign Study Roles to Assessment Definitions from EDC Tools > Assessments |
If your Study contains restricted data, you must have the Restricted Data Access permission to view it.
Learn more about Study Roles.
Viewing Assessments
Once you navigate to EDC Tools > Assessments, Vault displays a list of all existing Assessment Definitions in your study. You can filter this list by Type (from Assessment Type) or search for a specific Assessment. Before any Assessments display in this listing, a study designer must create them in Studio.
Assigning Assessments
You can assign each Assessment Definition to a Study Role. Users with this Study Role are able to view and perform any Assessments of that definition. For example, you may assign a Serious Adverse Event assessment to users with a custom SAE Assessment Editor role. Veeva has certain recommendations for managing Study Roles with assessments. See details below.
To assign an Assessment:
- Navigate to Tools > EDC Tools > Assessments for your Study
- Locate the Assessment that you want to assign a role to in the listing.
- Hover over that Assessment to display the Actions menu.
-
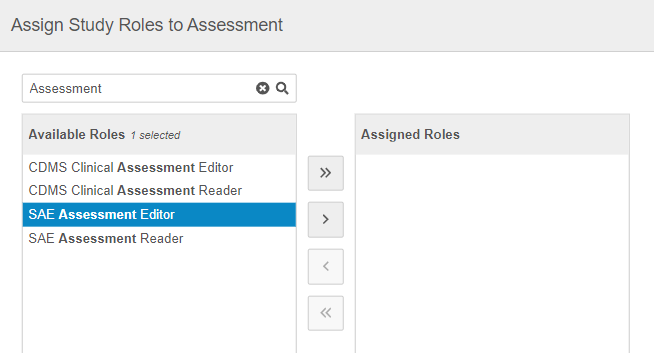
In the Assign Study Roles to Assessment dialog, search or scroll to locate the roles you want to assign to an Assessment.
-
Use the Arrow buttons or double-click on Study Roles to move them from the Available Roles column to the Assigned Roles column.
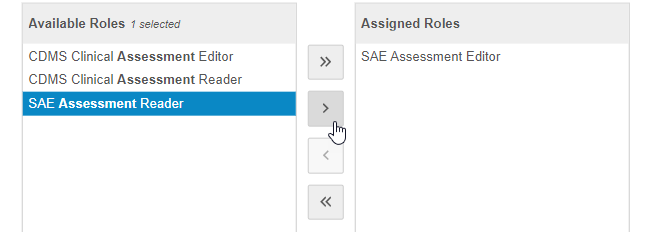
- When finished, click Save. Vault assigns the Assessment to the Study Roles in the Assigned Roles column.
Role Management for Assessments
Veeva recommends that you use the standard CDMS Clinical Assessment Reader and CDMS Clinical Assessment Editor study roles as templates to create custom Study Roles associated with each of your Assessment Definitions. For example, if you have a Serious Adverse Event Assessment, create an SAE Assessment Reader and an SAE Assessment Editor role for that assessment.
Learn more about creating custom Study Roles here.
