Standard CDMS Report Templates
Veeva EDC leverages the Vault Platform to provide reporting capabilities for operational metrics. Veeva provides standard report templates that you can create reports from by copying and configuring the columns and filters as needed.
If you open a linked object record from a report, Veeva EDC opens that object record in the related area of Veeva EDC, not in an object record Details page. See Sharing Settings for information on sharing reports.
Availability: Reporting functionality related to documents is not available in Veeva EDC.
Veeva Standard Report Template Policy
Standard report templates represent how Veeva EDC would display operational metrics. These standard templates provide a reference for custom reports customers may require in the conduct of their study. Standard report templates are locked to all users. If a customer would like to use a template in the execution of their study, they must create a copy of the template. Versions of the standard template reports are visible in the description.
Standard report templates are not supported as part of the contract with Veeva EDC and are subject to change. Therefore, customers should not base their standard operating procedures on the provided templates.
Label and Short Label Columns: Label and Short Label columns will only be filled for Form Definitions created after the 23R3 release. These columns will be blank for already existing Form Definitions.
Available Templates
Event Date field on the Event Operational Summary object: The Event Date field on the Event Operational Summary object should no longer be used in reports after the 25R1 release. Any reports using this field should be updated to pull the Event Date from the Event object instead.
The following standard report templates are available in Veeva EDC:
| Template | Description | Report Type | Data Model V1 Support | Data Model V2 Support | Notes |
|---|---|---|---|---|---|
| Site Performance | |||||
| Standard Template: Casebook Signature Summary | Summary of Casebooks with signatures | Casebook Operational Summary with Site | No | Yes | New in 20R2, for Data Model V2 studies. |
| Standard Template: Casebook Signature Summary (V3) | A summary matrix report of signature status by Casebook. Possible values include Yes to indicate if a casebook is fully signed and No to indicate if a casebook is not signed. | Casebook Operational Summary with Site | No | Yes | New in 23R3. It is recommended that you use this report version. |
| Standard Template: Frozen Casebook Summary | Summary of frozen Casebooks | Casebook Operational Summary with Site | No | Yes | New in 20R2, for Data Model V2 studies. |
| Standard Template: Frozen Casebook Summary (V3) | A summary matrix report of frozen status by Casebook. Possible values include Yes to indicate if a casebook is fully frozen and No to indicate if a casebook is not frozen. | Casebook Operational Summary with Site | No | Yes | New in 23R3. It is recommended that you use this report version. |
| Standard Template: Locked Casebook Summary | Summary of locked Casebooks | Casebook Operational Summary with Site | No | Yes | New in 20R2, for Data Model V2 studies. |
| Standard Template: Locked Casebook Summary (V3) | A summary matrix report of locked status by Casebook. Possible values include Yes to indicate if a casebook is fully locked and No to indicate if a casebook is not locked. | Casebook Operational Summary with Site | No | Yes | New in 23R3. It is recommended that you use this report version. |
| Standard Template: Event Status | Total Events by Status and Site | Template: Event with Event Definitions and Study and Site and Subject | Yes | No | For Data Model V2 studies: Use Event Status field from the Event Operational Summary object, rather than the Event object. |
| Standard Template: Event Status (V3) | A matrix report, filtered by Study, providing a count of events by site and status | Template: Event with Event Operational Summary | No | Yes | New in 23R3. It is recommended that you use this report version. |
| Standard Template: Event Signature Summary | Summary of Events with signatures | Template: Event with Event Definitions and Study and Site and Subject | No | Yes | New in 20R2, for Data Model V2 studies. |
| Standard Template: Event Signature Summary (V3) | A summary matrix report counting the signature status submitted events. Possible values include Yes to indicate if an event is signed, No to indicate if an event was signed and was unsigned, or None to indicate if an event has never been signed. | Event with Study Site and Event Operational Summary | No | Yes | New in 23R3. It is recommended that you use this report version. |
| Standard Template: Form Cycle Time Summary | Summary of Form cycle time metrics | Template: Form Cycle Time Summary | Yes | Yes | |
| Standard Template: Form Cycle Time Summary (V3) | A tabular report of form cycle times, broken down by site. Cycle times include: Visit to Form Submit, Form Submit to SDV, DMR, Signature, Frozen and Lock | Template: Form Cycle Time Summary | Yes | Yes | New in 23R3. It is recommended that you use this report version. |
| Standard Template: Form SDV Summary | Summary of source data verified Forms | Template: Study Site with Form | Yes | No | Data Model V2 studies should use V2 of this report. |
| Standard Template: Form SDV Monitoring Activity per Week | Summary of Form SDV monitoring activity per week by Site | Template: Form with Event and Study Site and Subject | Yes | No | Data Model V2 studies should use V2 of this report. |
| Standard Template: Form Signature Summary | Summary of signed Forms | Template: Study Site with Form | Yes | No | Data Model V2 studies should use V2 of this report. |
| Standard Template: Form Signature Summary (v2) | Summary of signed Forms | Template: Study Site with Form | No | Yes | Data Model V1 studies should use Standard Template: Form Signature Summary |
| Standard Template: Form Signature Summary (V3) | A summary matrix report counting the signature status submitted forms. Possible values include Yes to indicate if a form is signed, No to indicate if a form was signed and was unsigned, or None to indicate if a form has never been signed. | Form with Study Site and Summary | No | Yes | New in 23R3. It is recommended that you use this report version for Data Model V2 studies. |
| Standard Template: Form Status | Summary report of Site-level Form status | Template: Form with Event and Study Site and Subject | Yes | Yes | |
| Standard Template: Form Status (V3) | A summary matrix report of all form statuses, broken down by site and subject. | Template: Form with Event and Study Site and Subject | Yes | Yes | New in 23R3. It is recommended that you use this report version. |
| Standard Template: Frozen Form Summary | Summary of frozen Forms | Template: Study Site with Form | Yes | No | Data Model V1 studies should use V1 of this report (Standard Template: Frozen Form Summary) |
| Standard Template: Frozen Form Summary (v2) | Summary of frozen Forms | Template: Study Site with Form | No | Yes | Data Model V1 studies should use V1 of this report. |
| Standard Template: Frozen Form Summary (V3) | A summary matrix report counting the freeze status of submitted forms. Possible values include Yes to indicate if a form is frozen, No to indicate if a form was frozen and was unfrozen, or None to indicate if a form has never been frozen. | Form with Study Site and Summary | No | Yes | New in 23R3. It is recommended that you use this report version. |
| Standard Template: Frozen Event Summary | Summary of frozen Events | Template: Event with Event Definitions and Study and Site and Subject | Yes | No | Data Model V1 studies should use V1 of this report (Standard Template: Frozen Event Summary) |
| Standard Template: Frozen Event Summary (v2) | Summary of frozen Events | Template: Event with Event Definitions and Study and Site and Subject | No | Yes | Data Model V1 studies should use V1 of this report. |
| Standard Template: Frozen Event Summary (V3) | A summary matrix report counting the freeze status of submitted events. Possible values include Yes to indicate if an event is frozen, No to indicate if an event was frozen and was unfrozen, or None to indicate if an event has never been frozen. | Event with Study Site and Event Operational Summary | No | Yes | New in 23R3. It is recommended that you use this report version for Data Model V2 studies. Data Model V1 studies should use V1 of this report. |
| Standard Template: Locked Form Summary | Summary of locked Forms | Template: Study Site with Form | Yes | No | Data Model V1 studies should use V1 of this report (Standard Template: Locked Form Summary) |
| Standard Template: Locked Form Summary (v2) | Summary of locked Forms | Template: Study Site with Form | No | Yes | Data Model V1 studies should use V1 of this report. |
| Standard Template: Locked Form Summary (V3) | A summary matrix report counting the lock status of submitted forms. Possible values include Yes to indicate if a form is locked, No to indicate if a form was locked and was unlocked, or None to indicate if a form has never been locked. | Form with Study Site and Summary | No | Yes | New in 23R3. It is recommended that you use this report version for Data Model V2 studies. Data Model V1 studies should use V1 of this report. |
| Standard Template: Locked Event Summary | Summary of Locked Events | Template: Event with Event Definitions and Study and Site and Subject | Yes | No | Data Model V2 studies should use V2 of this report. |
| Standard Template: Locked Event Summary (v2) | Summary of Locked Events | Template: Event with Event Definitions and Study and Site and Subject | No | Yes | Data Model V1 studies should use V1 of this report (Standard Template: Locked Event Summary) |
| Standard Template: Locked Event Summary (V3) | A summary matrix report counting the lock status of submitted events. Possible values include Yes to indicate if an event is locked, No to indicate if an event was locked and was unlocked, or None to indicate if an event has never been locked. | Event with Study Site and Event Operational Summary | No | Yes | New in 23R3. It is recommended that you use this report version for Data Model V2 studies. Data Model V1 studies should use V1 of this report. |
| Standard Template: Query Aging by Site | Summary of query counts by Site and Query Age (<1 Day, 1-3 Days, 3-5 Days, 5-7 Days, >7 Days) | Template: Query Cycle Time Summary | Yes | Yes | |
| Standard Template: Query Aging by Site (V3) | A summary matrix report of the open and answered query counts by query age grouping (1 Week, 2 Week, 3 Week, 4 Week, >4 Weeks), grouped by site. | Template: Query Cycle Time Summary | Yes | Yes | New in 23R3. It is recommended that you use this report version. |
| Standard Template: Query Cycle Time Summary | Summary of query cycle time metrics to assess Sites on responsiveness and data conflict resolution | Template: Query Cycle Time Summary | Yes | Yes | |
| Standard Template: Query Cycle Time Summary (V3) | A tabular report of all queries in a study. Cycle times include Query Age, Time to First Response and Time from Open to Closed. | Template: Query Cycle Time Summary | Yes | Yes | New in 23R3. It is recommended that you use this report version. |
| Standard Template: Query Volume per Form | Number of query executions on a specific Form design (Form Definition) across a Study | Template: Form with Form Definition and Query Binding | Yes | Yes | |
| Standard Template: Query Volume per Form (V3) | A summary matrix report of the number of queries per form definition. | Template: Form with Form Definition and Query Binding | Yes | Yes | New in 23R3. It is recommended that you use this report version. |
| Standard Template: Query Volume per Week by Site | Number of queries generated across Sites in the Study each week | Template: Study Site with Query Binding | Yes | Yes | |
| Standard Template: Query Volume per Week by Site (V3) | A summary matrix report of the number of queries created per week, grouped by site. | Template: Study Site with Query Binding | Yes | Yes | New in 23R3. It is recommended that you use this report version. |
| Standard Template: Site Queries | Summary report of query count based on status at each Site in the Study | Template: Study Site with Query Binding | Yes | Yes | |
| Standard Template: Site Queries (V3) | A summary matrix report of the number of queries, grouped by query status and site. | Template: Query Binding with Study Site | Yes | Yes | New in 23R3. It is recommended that you use this report version. |
| Standard Template: Cumulative Subject Creation per Week | Cumulative Subject creation within the EDC system for each Site | Template: Subject | Yes | Yes | |
| Standard Template: Cumulative Subject Creation per Week (V3) | A summary matrix report of the number of subjects created per week, grouped by site. | Template: Subject | Yes | Yes | New in 23R3. It is recommended that you use this report version. |
| Standard Template: Subject Study Progression | Summary of a Subject’s progression through the Study | Template: Event with Event Definitions and Study and Site and Subject | Yes | No | |
| Standard Template: Subject Study Progression (V3) | A tabular report of planned and entered events, grouped by site and subject. | Template: Event Operational Summary | No | Yes | |
| Standard Template: Subject Study Progression (V4) | A tabular report of planned and entered events, grouped by site and subject. | Template: Event with Event Operational Summary | No | Yes | New in 25R1. It is recommended that you use this report version for Data Model V2 studies. |
| Standard Template: Subject Summary | Number of Subjects in each Status | Template: Subject with Study and Study Site | Yes | Yes | |
| Standard Template: Subject Status Summary by Site (V3) | A summary matrix report counting the number of subjects in each status, grouped by site. | Template: Subject with Study and Study Site | Yes | Yes | New in 23R3. This report template replaces Standard Template: Subject Summary. It is recommended that you use this report version. |
| Standard Template: Unique CRF Listing | Listing of unique (per Subject) Forms (CRFs) by Study | Template: Form Definition | Yes | Yes | |
| Standard Template: Unique CRF Listing (V3) | A tabular report of unique CRF definitions in a study. | Template: Form Definition | Yes | Yes | New in 23R3. It is recommended that you use this report version. |
| Study Closeout Status | Listing of Sites and their Site Closeout Status by Study | Template: Study Closeout | Yes | Yes | |
| Study Closeout Status (V3) | A tabular report of the closeout status of each site in a study. | Template: Study Closeout | Yes | Yes | New in 23R3. It is recommended that you use this report version. |
| Actionable | |||||
| Standard Template: Detailed Missing Forms Report | Listing of Forms missing per Site and Subject | Template: Form with Event and Study Site and Subject | Yes | Yes | |
| Standard Template: Detailed Missing Forms Report (V3) | A tabular report of non-submitted forms that are overdue. Please note this only includes forms that have been added to the schedule but are not yet submitted. Events must be configured with Overdue Days in order for forms at that event to be marked as overdue. | Template: Form with Event and Form Def, Site and Subject | Yes | Yes | New in 23R3. It is recommended that you use this report version. |
| Standard Template: Form SDV Detail | Detailed listing of Form SDV status | Template: Form with Event and Study Site and Subject | Yes | No | Data Model V2 studies should use V2 of this report. |
| Standard Template: Form DMR Summary | Summary listing of Form DMR status by Study Site | Template: Study Site with Form | Yes | No | Data Model V2 studies should use V2 of this report. |
| Standard Template: Items Intentionally Left Blank | Listing of Items in subject Casebooks that a data entry user marked as Intentionally Left Blank | Template: Item with Item History | Yes | No | Using Item2 as the primary reporting object isn't a supported configuration. |
| Standard Template: Items Intentionally Left Blank (V3) | A tabular report of items marked as intentionally left blank, grouped by site and subject and filtered by study. Only for studies on Data Model 2. | Template: Item 2 | No | Yes | |
| Standard Template: Query Details by Site and Subject | Detailed listing of queries per Subject | Template: Query with Subject and Query Binding and Query Message | Yes | Yes | |
| Standard Template: Query Details by Site and Subject (V3) | A tabular report of query details, grouped by site and subject. | Template: Query with Subject and Query Binding and Query Message | Yes | Yes | New in 23R3. It is recommended that you use this report version. |
| Standard Template: Comprehensive Form Summary | Detailed listing of submitted Forms and Form Review Status by Study Site and Subject | Template: Form with Event and Site and Subject | Yes | No | Data Model V2 studies should use V2 of this report. |
| Standard Template: Comprehensive Form Summary (v2) | Detailed listing of submitted Forms and Form Review Status by Study Site and Subject | Template: Form with Event and Site and Subject | No | Yes | Data Model V1 studies should use V1 of this report (Standard Template: Comprehensive Form Summary) |
| Standard Template: Comprehensive Form Summary (V3) | A tabular report detailing the forms' SDV, DMR, Frozen, Signature and Locked statuses and the dates when those were completed, grouped by site and subject. | Template: Form with Event, Form Def, Site, Subject, Form Summary | No | Yes | New in 23R3. It is recommended that you use this report version for Data Model V2 studies. Data Model V1 studies should use V1 of this report (Standard Template: Comprehensive Form Summary) |
| Study Progress Listing | |||||
| Template: Event Progress Listing | Listing of all Events and their progress in the Study | Template: Event Progress Listing | Yes | Yes | For Data Model V1 studies, some columns will be blank |
| Standard Template: Event Progress Listing (V2) | A tabular report with operational data at the form level, filtered by study. Report will only populate with data when a Event Progress Listing job is scheduled from EDC Tools. | Event Progress Listing with Listing Last Run | Yes | Yes | For Data Model V1 studies, some columns will be blank |
| Template: Form Progress Listing | Listing of all Forms and their progress in the Study | Template: Form Progress Listing | Yes | Yes | |
| Standard Template: Form Progress Listing (V2) | A tabular report with operational data at the form level, filtered by study. Report will only populate with data when a Form Progress Listing job is scheduled from EDC Tools. | Form Progress Listing with Listing Last Run | Yes | Yes | |
| Template: Query Detail Listing | Detailed listing of all Queries in the Study | Template: Query Detail Listing | Yes | Yes | |
| Standard Template: Query Detail Listing (V2) | A tabular report with operational data at the form level, filtered by study. Report will only populate with data when a Query Detail Listing job is scheduled from EDC Tools. | Template: Query Detail Listing with Listing Last Run | Yes | Yes | |
| Template: Subject Progress Listing | Listing of all Subjects and their progress in the Study | Template: Subject Progress Listing | Yes | Yes | |
| Standard Template: Subject Progress Listing (V2) | A tabular report with operational data at the form level, filtered by study. Report will only populate with data when a Subject Progress Listing job is scheduled from EDC Tools. | Template: Subject Progress Listing with Listing Last Run | Yes | Yes | |
| Coder | |||||
| WHODrug Coding Report | Clinical Coding Request and assigned codes (if assigned) for WHODrug based forms | Template: Clinical Coding | Yes | Yes | On vaults created before 23R3 |
| Standard Template: WHODrug Coding Report (V3) | Clinical Coding Request and assigned codes (if assigned) for WHODrug based forms; Standard Reports are updated by Veeva | Template: Clinical Coding | Yes | Yes | |
| Standard Template: WHODrug Coding Report (V4) | Clinical Coding Requests and assigned codes (if assigned) for WHODrug based forms. Includes Coding Notes and Queries. | Template: Clinical Coding with Notes and Queries | Yes | Yes | |
| Standard Template: JDrug Coding Report (V3) | Clinical Coding Request and assigned codes (if assigned) for JDrug based forms; Standard Reports are updated by Veeva | Template: Clinical Coding | Yes | Yes | |
| Standard Template: JDrug Coding Report (V4) | Clinical Coding Requests and assigned codes (if assigned) for JDrug based forms. Includes Coding Notes and Queries. | Yes | Yes | ||
| MedDRA Coding Report | Clinical Coding Request and assigned codes (if assigned) for MedDRA based forms | Template: Clinical Coding | Yes | Yes | On vaults created before 23R3 |
| Standard Template: MedDRA Coding Report (V3) | Clinical Coding Request and assigned codes (if assigned) for MedDRA and MedDRAJ based forms; Standard Reports are updated by Veeva | Template: Clinical Coding | Yes | Yes | |
| Standard Template: MedDRA Coding Report (V4) | Clinical Coding Requests and assigned codes (if assigned) for MedDRA and MedDRAJ based forms. Includes Coding Notes and Queries. | Template: Clinical Coding with Notes and Queries | Yes | Yes | |
| Operational | |||||
| Standard Template: Overdue Form Entry per Event | Summary of Events with overdue data entry tasks, including estimated Form workload | Template: Event with Event Operational Summary | Yes | No | Data Model V2 studies: Use the Event Status field from the Event Operational Summary object instead of the Event object |
| Standard Template: Overdue Form Entry per Event (V3) | A tabular report of blank and in progress events, grouped by site and subject. Includes the expected number of forms at each event and the number of days data entry is overdue. This report will only include events that are configured with an overdue days value. | Template: Event with Event Definition and Event Summary | Yes | No | New in 23R3. It is recommended that you use this report version. For Data Model V2 studies: Use the Event Status field from the Event Operational Summary object instead of the Event object |
| Standard Template: Schedule Deviation Report | Summary of Study Site and Subject adherence to the planned visit schedule per Study protocol | Template: Event Operational Summary | Yes | Yes | |
| Standard Template: Schedule Deviation Report (V3) | A tabular report of events, showing adherence to the planned visit schedule, grouped by site and subject. | Template: Event Operational Summary with Event Definition | Yes | Yes | New in 23R3. It is recommended that you use this report version. |
| Standard Template: Queries Leading to Data Changes | Summary list of system-generated Queries that lead to data changes | Query Operational Summary with Query | Yes | Yes | |
| Standard Template: Queries Causing Data Value Change (V3) | A tabular report of system queries that caused the item value to change, grouped by study and query rule. | Query Operational Summary with Query | Yes | Yes | New in 23R3. This report replaces Standard Template: Queries Leading to Data Changes. It is recommended that you use this report version. |
| Study Design | |||||
| Standard Template: Form Reason for Change Report | Summary count of reasons for post-submit Formchanges | Template: Item with Item History | Yes | No | Using Item2 as the primary reporting object isn't a supported configuration. |
| Standard Template: Item Reason for Change Report (V3) | A matrix summary report, counting the number of items per site updated with a specific change reason, filtered by study. Displays the latest change reason for an item. Does not include any system-managed change reasons. Only for studies on Data Model 2. | Template: Item 2 | No | Yes | |
| Standard Template: Query Volume by Rule Definition | Number of query executions for each Rule Definition | Template: Rule Definition with Study and Query Binding | Yes | Yes | |
| Standard Template: Query Volume by Rule Definition (V3) | A summary matrix report of the number of queries per rule definition, grouped by site. | Template: Rule Definition with Study and Query Binding | Yes | Yes | New in 23R3. It is recommended that you use this report version. |
| Standard Template: Unique Rule Definition Listing | Listing of unique Rules in a Study | Template: Rule Definition with Study and Query Binding | Yes | Yes | |
| Standard Template: Unique Rule Definition Listing (V3) | A tabular report of unique rule definitions. | Template: Rule Definition with Study and Query Binding | Yes | Yes | New in 23R3. It is recommended that you use this report version. |
| Review (For Studies created after the 19R3 release) | |||||
| Standard Template: Form SDV Monitoring Activity per Week (v2) | Summary of Form SDV monitoring activity per week by site | Template: Form with Event, Study Site, Subject, Form Summary | No | Yes | Data Model V1 studies should use V1 of this report (Standard Template: Form SDV Monitoring Activity Per Week) |
| Standard Template: Form SDV Monitoring Activity per Week (V3) | A summary matrix report of the number of forms marked as SDV Complete, per week. Sites are included when at least one form has been marked as SDV Complete. | Template: Form Operational Summary with Form and Study Site | No | Yes | New in 23R3. It is recommended that you use this report version for Data Model V2 studies. Data Model V1 studies should use V1 of this report (Standard Template: Form SDV Monitoring Activity Per Week) |
| Standard Template: Form DMR Monitoring Activity per Week (v2) | Summary of Form DMR monitoring activity per week by site | Template: Form with Event, Study Site, Subject, Form Summary | No | Yes | Data Model V1 studies should use V1 of this report (Standard Template: Form DMR Monitoring Activity Per Week) |
| Standard Template: Form DMR Monitoring Activity per Week (V3) | A summary matrix report of the number of forms marked as DMR Complete, per week. Sites are included when at least one form has been marked as DMR Complete. | Template: Form Operational Summary with Form and Study Site | No | Yes | New in 23R3. It is recommended that you use this report version for Data Model V2 studies. Data Model V1 studies should use V1 of this report (Standard Template: Form DMR Monitoring Activity Per Week) |
| Standard Template: Form SDV Summary (v2) | Summary listing of Form SDV status | Template: Form with Study Site and Summary | No | Yes | Data Model V1 studies should use V1 of this report (Standard Template: Form SDV Summary) |
| Standard Template: Form SDV Summary (V3) | A summary matrix report of SDV status at the form level. This only includes forms where SDV is required. Possible values: Yes to indicate if all required items on the form are SDV Complete or No to indicate if not all required items are SDV Complete | Template: Form Operational Summary with Form and Study Site | No | Yes | Data Model V1 studies should use V1 of this report (Standard Template: Form SDV Summary) |
| Standard Template: Form DMR Summary (v2) | Summary listing of Form DMR status | Template: Form with Study Site and Summary | No | Yes | Data Model V1 studies should use V1 of this report (Standard Template: Form DMR Summary) |
| Standard Template: Form DMR Summary (V3) | A summary matrix report of DMR status at the form level. Only includes forms where DMR is required. Possible values include Yes to indicate if all required items on the form are DMR Complete or No to indicate if not all required items are DMR Complete. | Template: Form Operational Summary with Form and Study Site | No | Yes | Data Model V1 studies should use V1 of this report (Standard Template: Form DMR Summary) |
| Standard Template: Form SDV Detail (v2) | Detailed listing of Form SDV status by Site | Template: Form with Event, Study Site, Subject, Form Summary | No | Yes | Data Model V1 studies should use V1 of this report (Standard Template: Form SDV Detail) |
| Standard Template: Form SDV Detail (V3) | A tabular report of SDV status for forms where SDV is required, grouped by site and subject. Possible values include Yes to indicate if all required items on the form are SDV Complete or No to indicate if not all required items are SDV Complete. | Template: Form Operational Summary with Event and Form Definition | No | Yes | New in 23R3. It is recommended that you use this report version for Data Model V2 studies. Data Model V1 studies should use V1 of this report (Standard Template: Form SDV Detail) |
| Standard Template: Form DMR Detail (v2) | Detailed listing of Form DMR status by Site | Template: Form with Event, Study Site, Subject, Form Summary | No | Yes | Data Model V1 studies should use V1 of this report (Standard Template: Form DMR Detail) |
| Standard Template: Form DMR Detail (V3) | A tabular report of DMR status for forms where DMR is required, grouped by site and subject. Possible values include Yes to indicate if all required items on the form are DMR Complete or No to indicate if not all required items are DMR Complete. | Template: Form Operational Summary with Event and Form Definition | No | Yes | New in 23R3. It is recommended that you use this report version for Data Model V2 studies. Data Model V1 studies should use V1 of this report (Standard Template: Form DMR Detail) |
| Safety | |||||
| Safety Cases | Listing of Safety Cases | Template: Safety | Yes | Yes | |
| Standard Template: Safety Cases (V3) | A tabular report of safety cases in a study. | Template: Safety | Yes | Yes | New in 23R3. It is recommended that you use this report version. |
| Safety Messages | Listing of Safety Messages | Template: Safety | Yes | Yes | |
| Persona-Based Dashboards * These reports exist to power persona-based dashboards. |
|||||
| Dashboard: Out of Range Event Total | Number of out of range Events | Template: Event with Event Operational Summary | Yes | No | Data Model V2 studies: Use the Event Status field from the Event Operational Summary object instead of the Event object |
| Dashboard: Number of Enrolled Subjects | Number of enrolled Subjects by Study | Template: Subject with Study and Study Site | Yes | Yes | |
| Dashboard: Open Queries | Number of open queries | Template: Query Cycle Time Summary | Yes | Yes | |
| Dashboard: Answered Queries | Number of answered queries | Template: Query Cycle Time Summary | Yes | Yes | |
| Dashboard: Form Status (Not Completed) | Number of Forms not in the Completed status | Template: Form with Event and Study Site and Subject | Yes | Yes | |
| Dashboard: Query Volume by Form | Number of Queries by Form | Template: Form with Form Definition and Query Binding | Yes | Yes | |
| Labs | |||||
| Standard Template: Site Lab with Lab Location | Shows Lab Locations and the Sites using those locations | Template: Site Lab with Lab Location | Yes | Yes | |
| Standard Template: Lab Reference Ranges | Shows Lab Locations and the Normal Ranges they use | Template: Lab Location with Lab Reference Range | Yes | Yes | |
| Standard Template: Lab Item and Analyte Definitions | Shows Lab Item Definitions and Analyte Definitions used within a study. | Template: Item Definition with Analyte Definition | Yes | Yes | |
| Randomization | |||||
| Emergency Unblinding Report | Shows a list of all emergency unblinding actions performed for a study | Randomization Action History | Yes | Yes | |
| License Keys | |||||
| Standard Template: Study Masters and License Keys in EDC Tools | Lists all study masters and any license key values entered in EDC Tools | Template: Study Master with Study Master - License | The data in this report is visible to Vault Owners. | ||
Available Report Type Templates
The following standard Report Type templates are available within Veeva EDC.
| Name | Description | Primary Reporting Object | Related Objects | Application |
|---|---|---|---|---|
| Template: Event Operational Summary | This report type can track the number of expected forms, the number of days overdue the forms in the event are, the event date, and the number of days outside of the event window the event date is. | Event Operational Summary | N/A | EDC |
| Template: Event Progress Listing | This report type is created from the Event Progress Listing job in EDC Tools > Job Schedule. | Event Progress Listing | Event Progress Listing | EDC |
| Template: Event Progress Listing with Listing Last Run | This report type is created from the Event Progress Listing job in EDC Tools > Job Schedule. | Event Progress Listing | Event Progress Listing | EDC |
| Template: Event with Event Definitions and Study and Site and Subject | This report type can display information about Events with reference information about the event design, the Study, the Site, and the Subject associated with the Event. | Event |
|
EDC |
| Template: Event with Event Operational Summary | This report type can display the Event Operational Summary as well as event details like the review status, creation date, and the Planned Date. | Event | Event Operational Summaries | EDC |
| Template: Form Cycle Time Summary | This report type can list the average time taken for each form to be completed/submitted | Form Operational Summary | N/A | EDC |
| Template: Form Definition | This report type can display form definitions. | Form Definition | N/A | EDC |
| Template: Form Progress Listing | This report type is created from the Form Progress Listing job in EDC Tools > Job Schedule. | Form Progress Listing | Form Progress Listing | EDC |
| Template: Form Progress Listing with Listing Last Run | This report type is created from the Form Progress Listing job in EDC Tools > Job Schedule. | Form Progress Listing | Form Progress Listing | EDC |
| Template: Form with Event and Study Site and Subject | This report type can display information about Forms with reference information about SDV Details and Status | Form |
|
EDC |
| Template: Item 2 | This report type can list information about Items intentionally left blank and Item change reasons. | Item | N/A | EDC |
| Template: Query Cycle Time Summary | This report type can list the average time taken for each query to be answered or resolved | Query Operational Summary | N/A | EDC |
| Template: Query Detail Listing | This report type is created from the Query Detail Listing job in EDC Tools > Job Schedule. | Query Detail Listing | Query Detail Listing | EDC |
| Template: Query Detail Listing with Listing Last Run | This report type is created from the Query Detail Listing job in EDC Tools > Job Schedule. | Query Detail Listing | Query Detail Listing | EDC |
| Template: Query with Subject and Query Binding and Query Message | This report type can list Subjects, Sites, and information regarding Queries and Query Binding and Messages. | Query |
|
EDC |
| Template: Rule Definition with Study and Query Binding | This report type can list Rule Definitions as well as information regarding Name, Query Binding, and Study Site | Rule Definition |
|
EDC |
| Template: Study Site with Form | This report type can list information regarding the Study Site name and reference information about corresponding Forms. | Study Site | Forms | EDC |
| Template: Study Site with Query Binding | This report type can list information regarding Study Sites and the corresponding Queries. | Study Site | Query Bindings | EDC |
| Template: Subject | This report type can provide information regarding the subject. | Subject | N/A | EDC |
| Template: Subject Progress Listing | This report type is created from the Subject Progress Listing job in EDC Tools > Job Schedule. | Subject Progress Listing | Subject Progress Listing | EDC |
| Template: Subject Progress Listing with Listing Last Run | This report type is created from the Subject Progress Listing job in EDC Tools > Job Schedule. | Subject Progress Listing | Subject Progress Listing | EDC |
| Template: Subject with Study and Study Site | This report type can list information regarding Subject Status and Study Site. | Subject |
|
EDC |
| Template: Safety | This report type can list information about Safety Cases and Safety Messages, with context from the Safety Case Form. | Safety Case |
|
EDC, Safety |
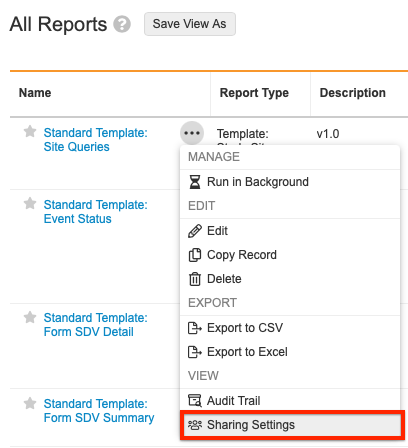
Sharing Settings
You must add the appropriate users to the report sharing settings for each new report version.
To access report sharing settings, click the Actions button next to the report you’d like to update in the Reports tab.
Related Permissions
Reporting functionality is available by default to users with the following standard study roles:
- CDMS Clinical Coder
- CDMS Clinical Coder Administrator
- CDMS Clinical Research Associate
- CDMS Data Manager
- CDMS Lead Data Manager
- CDMS Study Designer
- CDMS User Administrator
If your organization uses custom Study Roles, you must have the Reports Dashboards Tab and Schedule Reports permissions to use all reporting functionality.
Individual reports must be shared with Study Roles or with custom Groups.