New Features in 25R3 Limited Releases
25R3 Planned General Availability: November 21 & December 5, 2025
25R2.4 Release Date: October 17, 2025
25R2.3 Release Date: October 3, 2025
25R2.2 Release Date: August 22, 2025
We are pleased to bring you new functionality with each limited release. These release notes are updated with upcoming new features one week before the limited release date.
Enablement Changes: The enablement of each feature is subject to change from release to release. For limited releases in the same general release, we will update this page. Enablement may also change before the general release. Refer to the Release Impact Assessment for the most up to date enablement for a general release.
Clinical Data
Features in this section are changes that apply to all application areas of Veeva EDC and CDB.
Improved Multi-Vault User Experience 25R2.4
Use Case
When customers are working with more than one vault, users frequently have to switch between those vaults. For example, CRAs often require switching between a Veeva EDC vault and Veeva CTMS vault.
Prior to 25R3, if a user had logged in to Vault A in two tabs or windows, and they used the Vault Selector to change to a Vault B, they would be logged out from their Vault A in the other tab or window.
Now, Vault users can use the Vault Selector to change Vault applications without being logged out from other Veeva applications in different browser tabs or windows.
Description
When users are working with vaults in different browser tabs or windows, Vault sessions will remain active in all tabs if the user chooses the Vault Selector to change Vaults within a tab or window.
Enablement & Configuration
This feature is automatically enabled for all vaults.
Permission & Security Updates 25R2.3
Use Case
This update provides increased access to CDB users.
Description
This release includes updates to the following standard roles:
- A new permission, Upload 3rd Party Data Packages, has been added to the CDMS Lead Data Manager, CDMS Data Manager, CDB Data Provider, and CDMS Super User roles. This permission requires API Access.
- The View Protocol Deviations permission has been added to the CDMS CDB Read Only role.
Enablement & Configuration
These updates are automatically available for customers with CDB enabled.
Learn More
Data Entry
Features in this section are changes to the Data Entry tab, a working area for investigators and clinical research coordinators to enter study execution data.
Disable Bulk Signature When No Signature Definition Is Configured 25R2.2
Use Case
The behavior of the Bulk Sign Casebooks button and menu item have been updated to provide clarification to site users, reducing confusion and preventing issues when bulk signing Casebooks.
Description
In Data Entry, the Bulk Sign Casebooks button at the top right of the Subjects grid is disabled when there is no Casebook Signature Definition configured for the Study. When hovering over the disabled button, site users will see the message “This action cannot be performed because there is no signature definition for the study”. Similarly in the Site grid, the menu option will be grayed out and the same hoverover message will display.
Enablement & Configuration
This feature is automatically available.
Improved UI for Grid Views During Auto-save 25R2.2
Use Case
The user interface has been improved to reduce confusion and better support data entry for studies designed with a large number of items and item group repeats using the grid view. The auto-save feature had previously caused entered values to appear to be removed when the site user quickly tabbed and entered data into the next item. With the improved UI, it will be clearer to the Site users that the values within the grid view are being auto-saved.
Description
As Site users rapidly enter data, navigating the item repeats and tabbing very quickly across the grid view, the data they enter will be more visible as it auto-saves. While adding and editing items within the grid view, the value entered will appear grayed out (uneditable) as it auto-saves.
Enablement & Configuration
This feature is automatically available.
Item Groups and Items Created Concurrently with Forms 25R2.3
Use Case
Previously when a Form was added to a schedule, the system created a placeholder record (facade) of the Form in the administrative area. The item records on that Form had only been created when data was first auto-saved on the Form, either by entering data in an Item, opening a manual query, protocol deviation, freezing/locking etc. By creating the Item and Item Group records at the same time as the Form creation the timing and the change reasons of the Form, Item, and Item Group creations are reflected more accurately.
Description
When a Form is created, the creation records in the administration area for the Item Groups and Items on those Forms will also be created at the same time as the Form creation. In the audit trail, the creation datetime for Item Groups and Item records on the Form will be roughly the same as the Form creation datetime. Changes will be attributed to “System on behalf of {user}” for Form and Item creations, and the change reason “Changes prior to submission” will be included for Items with default data. Form creation can occur as part of the following actions and will reflect the user that performed the action:
- Entering an Event Date (Site user or API)
- Adding an Unscheduled event
- Dynamic rules
- Retrospective Amendment
- A repeating Form configured with a minimum number of form repeats
Enablement & Configuration
This feature is available for phased release. Contact Veeva Services for more information.
Pre-fill Lab Result Unit Based on Normal Range Unit 25R2.2
Use Case
Prior to this release, the system pre-filled the unit field for an analyte result with the standard Lab Unit Item Definition on that Lab Unit Definition when there is no normal range found. This feature reduces the risk of sites entering data with the incorrect unit.
Description
For lab analytes with the Unit data type, the unit of the Lab Result and Lab Normal Override (if configured) will be pre-set based on the below conditions:
- If there is a normal range value/unit available for this analyte, then the Lab Result unit and Lab Normal Override unit should be pre-set with the same unit in the normal range.
- If there is only one unit item definition for this unit definition, then the Lab Result unit and Lab Normal Override unit are pre-filled with this unit item definition.
- If there is no normal range value/unit available for this analyte, then the Lab Result unit and Lab Normal Override unit should be left blank and the user should need to select a unit for the lab result from the available options.
Enablement & Configuration
Auto-on.
Prevent Autosave of Clinical Significance Value When Not Enabled for Entry 25R2.2
Use Case
Previously, site users were able to enter Clinical Significance values even when the field was no longer applicable due to updated lab normal range indicators. This feature prevents this behavior from occurring.
Description
The system will block autosaving of the Clinical Significance value when the normal range flag value is null, Inconclusive, or Not Applicable.
Enablement & Configuration
This feature is immediately available in studies where lab clinical significance is enabled.
Show Mark Event as Did Not Occur for Confirm Value Queries on Blank Events 25R2.2
Use Case
This feature provides improved action buttons for events with quick queries. For special cases, such as an overdue or reset event, users will now see a more intuitive set of options. For example, if an event with a manual query is reset, the query remains on the event. The options displayed to the site will be to enter the event details, reply to the query, or mark the event as Did Not Occur, making it easier and more efficient to manage and resolve event queries.
Description
Confirm value quick queries showing on a blank event (for example, if the event was reset after the query was opened) now display an option for the site to mark the event as Did Not Occur, instead of Confirm Value.
Enablement & Configuration
This feature is automatically available upon release.
Data Review
Features in this section are changes to the Review tab, a working area for clinical research associates and data managers, or to review functionality within the Data Entry tab.
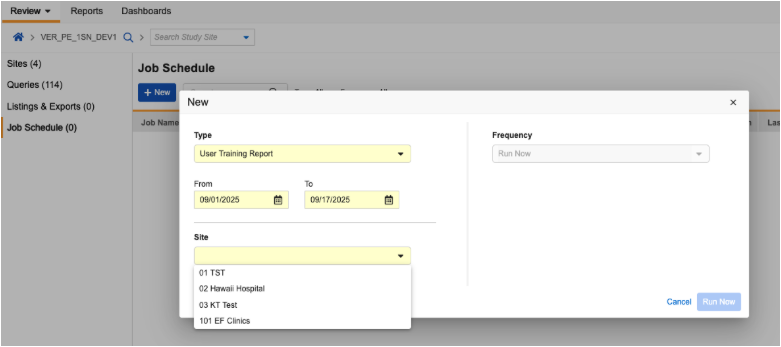
Site User Training Report for Review Users 25R2.3
Use Case
These updates expand access to Site User training information to CRAs and other Data Review users.
Description
The User Training Report is now available in the Review Tab under Listings and Exports. Users with the standard CDMS Clinical Research Associate or CDMS Data Manager roles, or a custom role with Review Tab access and the Manage Jobs permission, can now run the report.
The report runs for one site at a time and the user selects from sites that they have access to. The report shows training information for Site Users with access to the selected site.
Enablement & Configuration
These updates are immediately available.
Learn More
Clinical Coding
The following are new features for Veeva Coder, the clinical coding area for Veeva Coder.
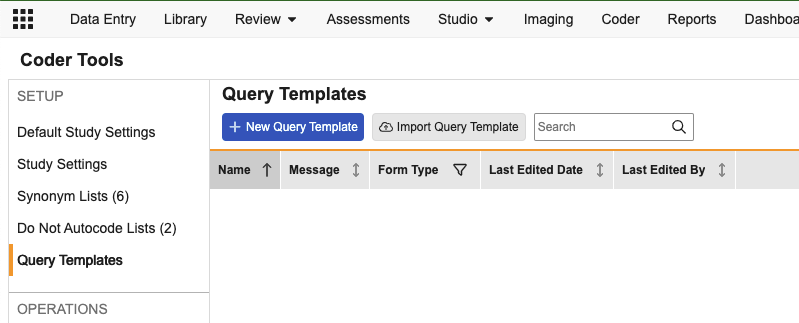
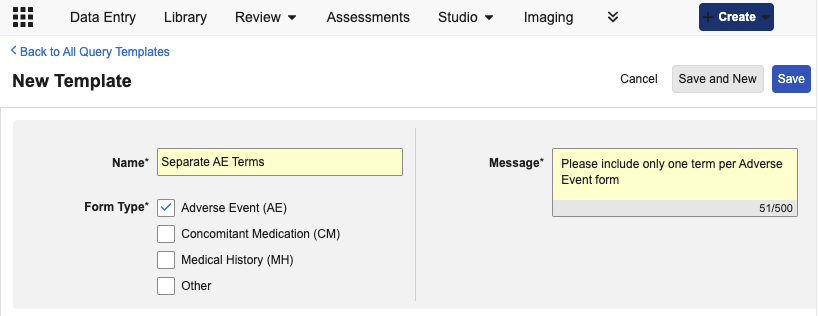
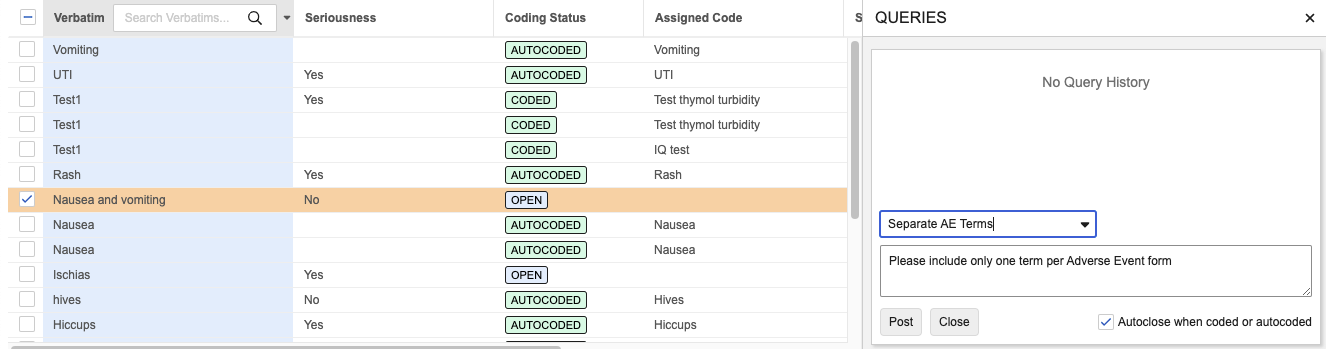
Coder: Query Templates 25R2.3
Use Case
This feature provides a faster and more controlled way for Coders to create queries for common issues.
Description
From the new Coder Tools > Query Templates subtab, Coder Administrators can add a new Query Template, specify a name for the template, the types of Coding forms it applies to, and the standard query text.
When issuing a query for a verbatim, Coders can use the searchable drop down menu to select from available templates for that form type.
The default query text for the template will populate, but can be customized as needed by the Coder before posting the query. Query templates can be exported from one vault and imported into another. Up to 20 templates are allowed for each coding form type.
As part of this feature, Coding Form Type labels have been updated across the UI and Study Design Specifications from AE, ConMed, and Medical History, to Adverse Event (AE), Concomitant Medication (CM), and Medical History (MH). Other remains unchanged.
Enablement & Configuration
This feature is automatically available for the General Release.
Coding Administration
The following are new features for Coder Tools, the administration area for Veeva Coder.
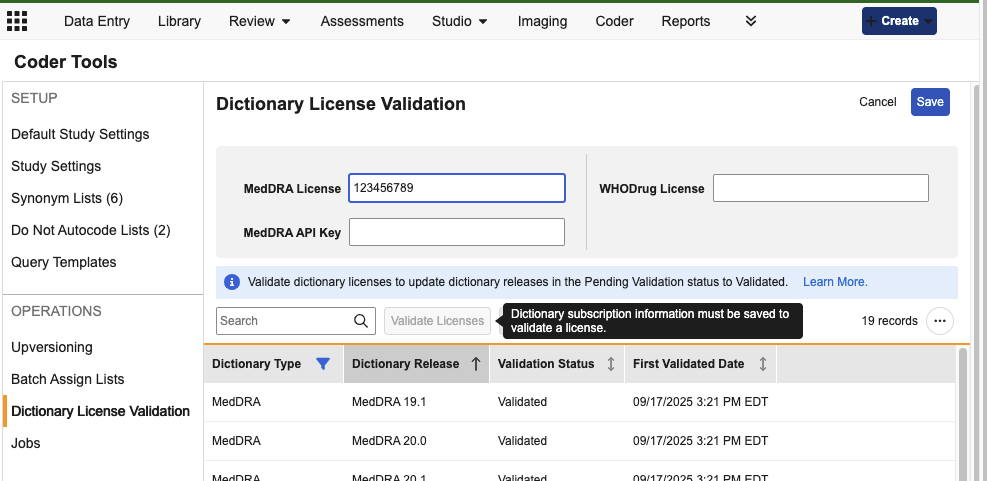
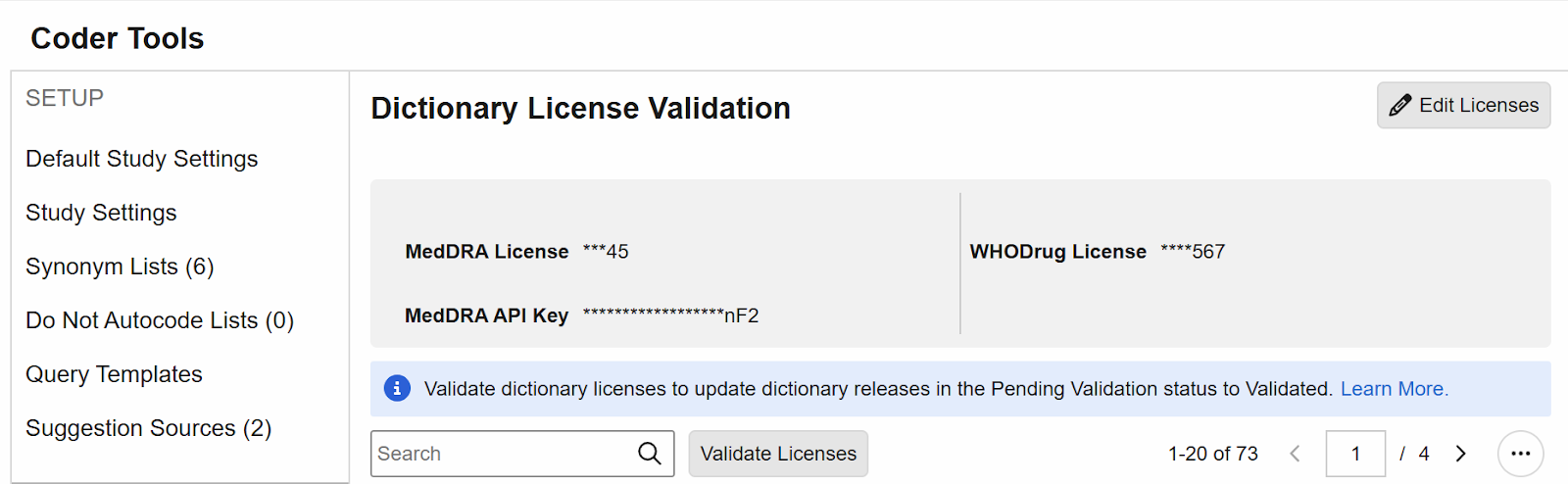
Validate Coding Dictionary Licenses 25R2.3
Use Case
This feature streamlines the process for ensuring customers are licensed for the coding dictionaries used in their studies.
Description
A new sub-tab in Coder Tools has been added for Dictionary License Validation. Coder Administrators and custom roles with the Manage Coder Study Settings permission have the ability to Edit License and API Key values and then subsequently Validate the information.
MedDRA and WHODrug versions that are in use or have been previously used will have the status of Validated at the time of the 25R3 release. Never-used and future new versions will have the status of Pending Validation, and license validation must occur before they can be assigned to a coding Form or Synonym List, or be used in a versioning change.
After providing the applicable license information and saving, the user can validate the licenses. For subsequent dictionary releases, users must return to Coder Tools > Dictionary License Validation to enter or edit and validate their licenses before the new dictionary version can be used.
When not in Edit mode, any saved values will show as partially masked to all users.
Note that this licensing feature does not apply to JDrug dictionaries, which will not show in this grid, and will be automatically available for use.
Enablement & Configuration
This feature is automatically available for the General Release.
Imaging
The following are new features for Veeva EDC Imaging, the imaging exam module for Veeva EDC.
Imaging: Dicom Pixel Masking 25R2.4
Description
Prior to uploading images, during their review and verification, site users with the Upload Exam permission can utilize new imaging tools to select and mask regions of pixels containing PHI/PII. While applying the masking, images within a DICOM series, that are the same size and resolution dimensions, will receive masking applied to the same positioning. If the images in the series have varying resolution dimensions, the system requires users to apply masks to the images individually unless the images that have different resolution dimensions are deleted. This automation saves effort for site users as they can apply the masking area once rather than to each of the images within a series. Masking can be resized and repositioned as needed. Messages and dialogs guide users as they are applying and adjusting the pixel masking.
After exam upload, the masking appears as solid black, fully redacting the underlying pixel data. The pixel data is removed from the images seen throughout EDC: Data Entry, Review, Assessments, Imaging and Vault Rendition Services (VRS). Masking is also maintained when using the panning and zooming features to view the images.
Site-level Image Redaction Setting
In EDC Tools, the New Study Site and Edit Study Site dialogs now include a new setting, Disable Image Redaction. The setting is defaulted to No and allows the site to utilize DICOM pixel masking to redact personal data (PHI/PII) from the images before uploading them to Data Entry.
The setting can also be applied to sites in bulk from the Sites grid. A new ‘Disable Image Redaction’ column will indicate the current image redaction status for the site and a new filter, Disable Image Redaction, is available to filter the grid records for all, disabled and enabled sites for image redaction.
To disable or enable Image Redaction in bulk, a new Disable Image Redaction button will appear once one or more sites are selected. If clicked and confirmed in the warning dialog, all selected sites will be disabled for image redaction. Alternatively, the Enable Image Redaction option can be chosen and will enable all selected sites for image redaction once the warning dialog is confirmed.
Locked sites will not be selectable to enable or disable image redaction. The Disable Image Redaction setting will also be supported in the site import file with allowed values Yes and No.
Enablement & Configuration
This update is automatically available for early adopters using Veeva EDC Imaging.
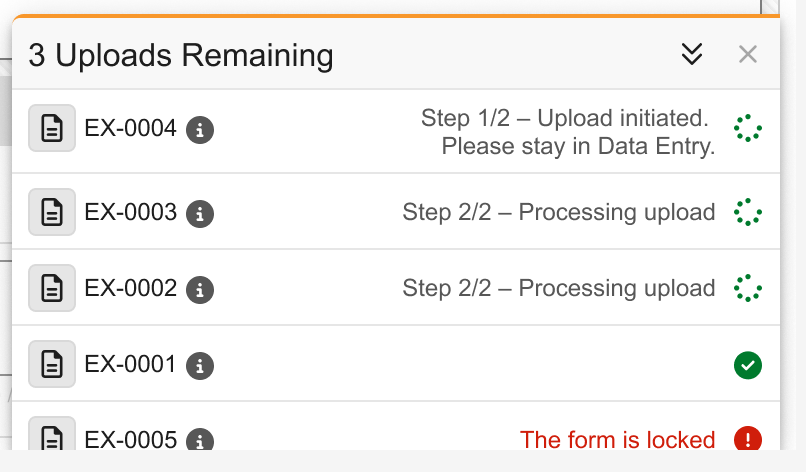
Imaging: Asynchronous Exam Upload Workflow 25R2.3
Use Case
While an exam upload is in process, users might want to perform other tasks or even log out of the system. The asynchronous processing of the exam upload will assure that the upload will finish in the background, even if not completed when leaving the upload screen. This improves the site user experience and allows for a more efficient data entry workflow with continued exam upload processing in parallel.
Description
In Data Entry, once the user starts an exam upload in the Imaging Exam Item Group and clicks Verify and Upload to start the exam upload, the system processes the exam asynchronously in the background. The user can now navigate away from the Form in Data Entry, refresh the browser, or log out from Veeva EDC without interrupting the upload process.
To further support the asynchronous process, the upload drawer displays the following messages when the upload is not yet started, and once the upload to the Veeva EDC system is in progress.
- Step 1/2 – Upload initiated. Please stay in Data Entry.
- Step 2/2 – Processing upload
If the user attempts to navigate away from the Data Entry Form, refresh the browser, or log out, a warning will only be shown while the exam upload is still in step 1 and has not yet started processing. The user can also start another exam upload while one or more exam uploads are already being processed asynchronously in the background.
When logging back into the system, the upload drawer expands and displays all unfinished exam uploads.
The system notifies the user via email upon successful completion or failure of an exam upload.
Enablement & Configuration
This update is automatically available for early adopters using Veeva EDC Imaging.
Assessments
The following are new features for the Assessments area of Veeva EDC.
Archive Choices for Assessments 25R2.3
Use Case
This updated functionality when configuring Assessments in Studio reflects similar functionality within the application, prevents duplicate choices, and helps prevent issues across environments in cases where configurations were removed in DEV.
Description
In Studio, when configuring Assessments, the Choice Item Definitions for Questions are no longer able to be removed if they were saved previously. Study designers can archive these when no longer needed. Users will only see the Remove button () for radio button and picklist choices when the selections have not yet been saved. The Inactive checkbox is now labeled Archive. To support this change, the dialog now includes a new filter, Show Archived Choices. Names of Choice Item Definitions will become read only once they are saved.
Additionally, column labels in the Assessments tab of Study Design Specifications (SDS) have been updated to match their applicable Assessment configuration areas:
- “Selection Name” is updated to “Choice Name”
- “Selection Label” is updated to “Choice Label”
- “Selection Inactive” is updated to “Choice Archived”
Enablement & Configuration
This feature is automatically available in Studio upon release.
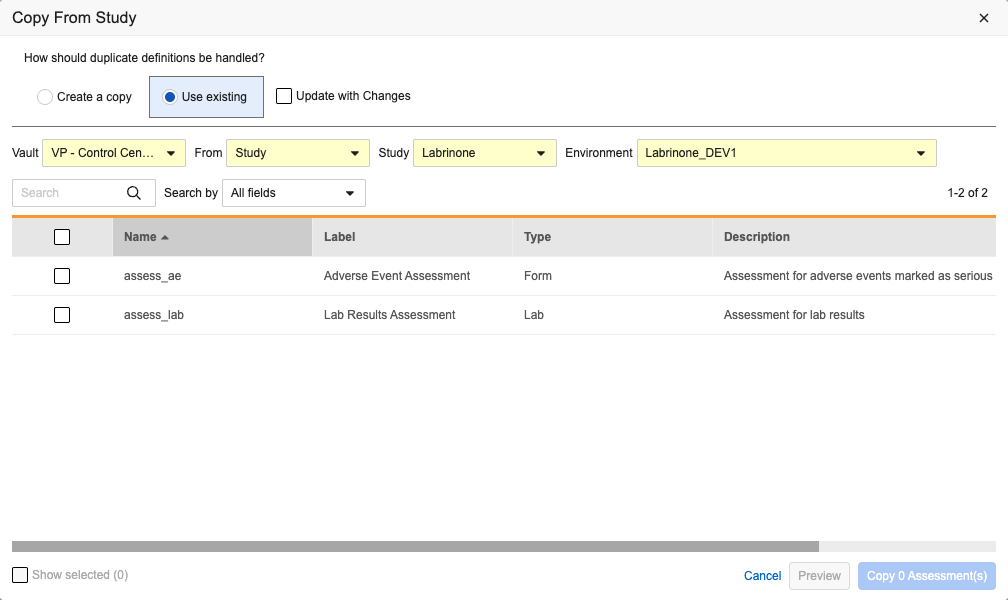
Copy Assessment Configurations 25R2.2
Use Case
The Copy Assessment Configurations feature further enhances Studio’s copy capabilities, increases study design reuse, and reduces efforts for study designers when creating assessments similar to other studies.
Description
Study designers can more easily configure assessments in Studio by copying assessment configurations from another study. The Copy From Study button has been added to the Assessments grid in Studio. Clicking the button opens the Copy From Study dialog where a study designer can select the Vault, the Study or Library, and the Environment that they want to copy from. Study designers can copy specific assessment configurations from within their current vault or from another vault and domain where the designer has access.
Similar to other Design Copy functionality, the dialog includes a search bar and has the following options for handling duplicate definitions:
- Create a copy
- Use existing and Update with Changes checkbox
Study designers can preview the results of their selections without actually copying the definitions into the study by clicking the Preview button.
Clicking the Preview and Copy Assessments buttons will run the applicable job and notify the user through email when the job completes. The email will confirm the job success or failure and includes a link to download the output file in .xlsx format.
The Copy Assessments job will handle supplemental data mismatches between the source and target study’s assessments, depending if the item exists or not and if the item is visible or hidden, providing warnings and status messages within the output file.
Enablement & Configuration
This feature is automatically available in Studio upon release.
Study Design & Configuration
Features in this area apply to Studio, the study design and configuration area for Veeva EDC.
Reset Forms with External Data & Repeating Item Groups 25R2.2
Use Case
External data within repeating item groups will no longer be removed when a form is reset. This allows sites to revise enterable data after a form has been reset, reducing reconciliation efforts and removing the need for third-party vendors to resend the data.
Description
When a site user resets a form, data loaded from APIs within repeating item groups remains on the form. External data displays in its original state, before any edits were made to the form.
Enablement & Configuration
This feature is automatically available for the general release.
Learn More
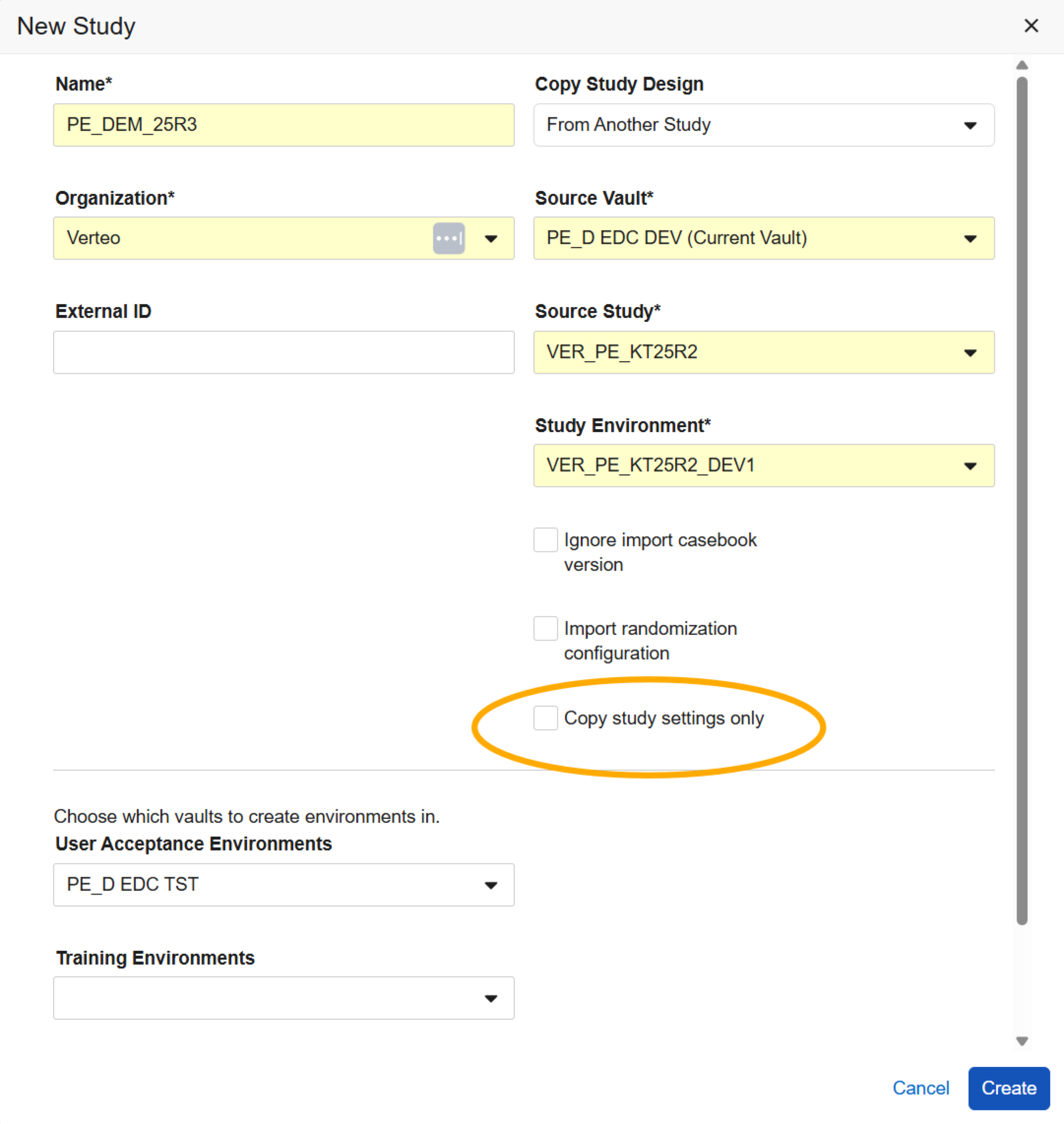
Copy Study Settings 25R2.2
Use Case
Study Designers can further reuse study configurations by copying Study Settings from other studies and libraries.
Description
When creating a study, the New Study dialog now includes an additional checkbox “Copy study settings only”. When this checkbox is selected, the system will only copy over the study settings from the selected source vault, Study/Library, and environment into the new study. When the checkbox is unselected, the system will copy over the study settings along with the study design configurations: Event Groups, Events, Forms, Item Groups, Items, Codelists, Rules, etc.
The study settings copied over will include the selections within the following:
- Studio > Study Settings
- Study Properties
- Study Phase
- Study Status
- Enforce Study Language
- Study Language
- Study Locale
- General
- Standard Date Format
- Twelve Hour Time
- Enable Other Specify - Reason for change
- Enable Other Specify - Intentionally Left Blank
- Enable Team Query Restrictions
- Query Team for System Queries
- Open Query on Out of Range Event Dates
- Enable Quick Queries
- Enable Veeva Randomization
- Enable Local Labs
- Safety Integrations Type
- Enable Protocol Deviations
- Enable Additive Review
- Enable Data Loader
- Enable Subject Status Date Rollback
- Enable Imaging
- Study Properties
- Default Configuration Options
- Signature Definitions
- Labs > Study General Settings (When Studio > Study Settings Enable Local Labs is Yes)
- Allow Site Overrides for Lab Normals
- Enable Pending Lab Locations
- Enable Lab Normal Entry in Form
- Default Day and Month for Age calculations
- Disable Unit selection for Lab Results when Lab Normals are present
- Lab Collection Time
- Fire Out-of-range Queries by Default
- Enable Query Lab Units Do Not Match
- Enable Query Lab Age Calculation
- Enable Query for Missing Lab Results
- Enable Clinical Significance
- Study Clinical Significance Codelist (copied when the Enable Clinical Significance setting is Yes)
When selecting “Copy study settings only”, the “Ignore import casebook version” and “Import randomization configuration” checkboxes will remain grayed out.
This option is only available at the time that the study is being created. After a study is created, the settings must be updated manually.
Enablement & Configuration
This feature is automatically available in Studio.
Cross-Form Derivations Update Code Requests 25R2.2
Use Case
This update allows study designers to use cross-form derivations to derive values used for Coding Configuration. For example, Indication can be derived to a Concomitant Medication form from a linked Adverse Event or Medical History form.
Description
With this release, a Code Request will update automatically if a cross-form derivation rule updates an item value included in the coding configuration, without requiring form resubmission. Note that if the coding form is in an unsubmitted state while the derived item value changes, the code request will not update until the form is submitted.
Prior to this release, cross-form derived items on Coding Forms could not effectively be included in coding configuration because Code Requests only update on form submission. Therefore, if the cross-form derived item was updated outside of a submission of the coding form, the updated information was not reflected in the Code Request.
Enablement & Configuration
This feature is immediately available.
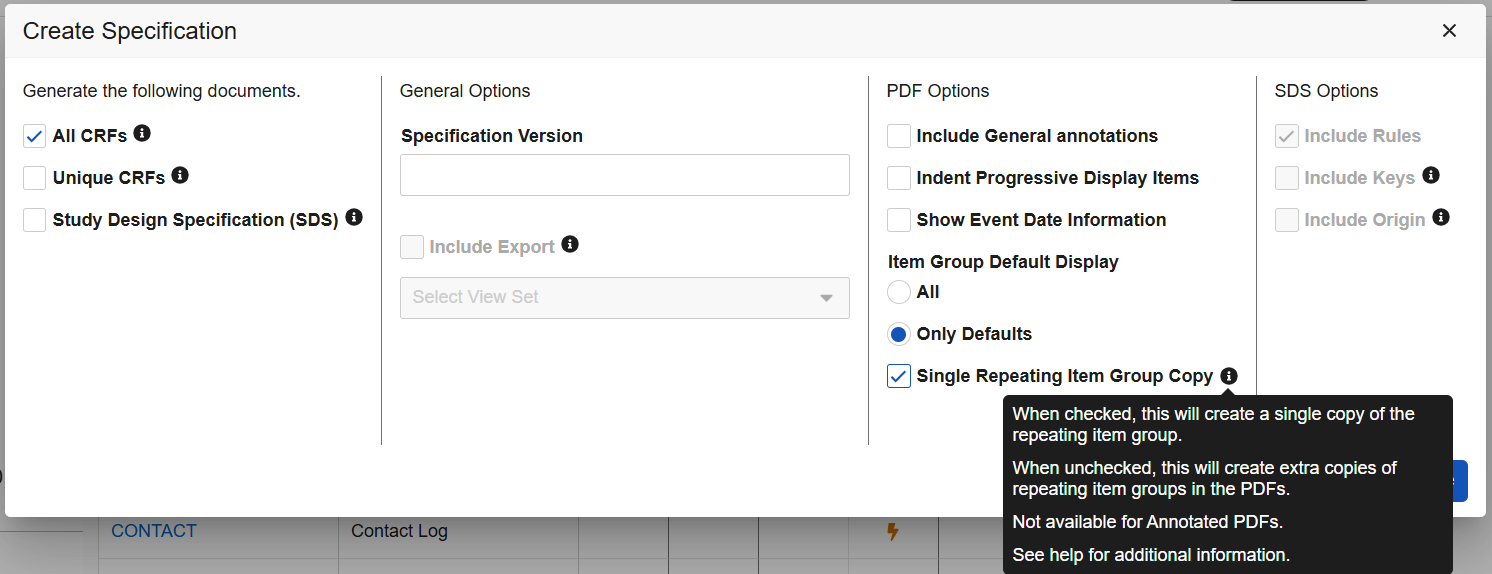
Studio: New Option for Displaying Default Data in Blank PDFs 25R2.3
Use Case
The Studio-generated Blank PDF includes a new option which improves how the PDF specifies forms with default data and visibility criteria.
Description
When creating specifications in Studio and selecting the option for either All CRFs or Unique CRFs, a new checkbox, Single Repeating Item Group Copy, appears within the PDF Options. The new setting includes an information icon with hoverover text.
When Single Repeating Item Group Copy is checked, a single copy of the repeating item group is included in the output blank PDF, listing the collective set of defaults.
An appendix within the PDF provides further specifications about the item group defaults and visibility criteria.
When unchecked, the PDFs will display the configured defaults and visibility criteria each as separate instances of the repeating Item Group. This option cannot be used when Include General Annotations is selected.
Enablement & Configuration
These changes are automatically available in Studio.
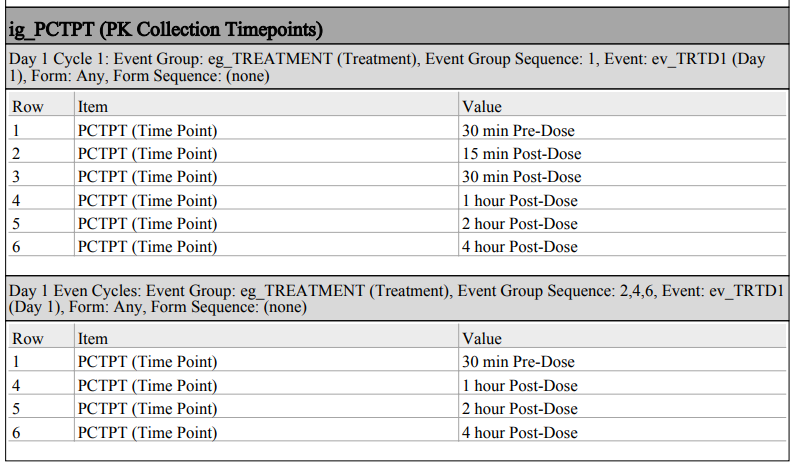
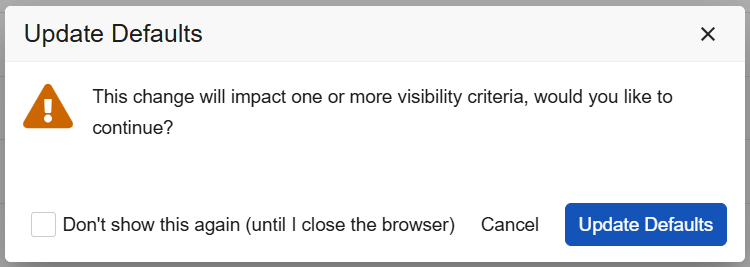
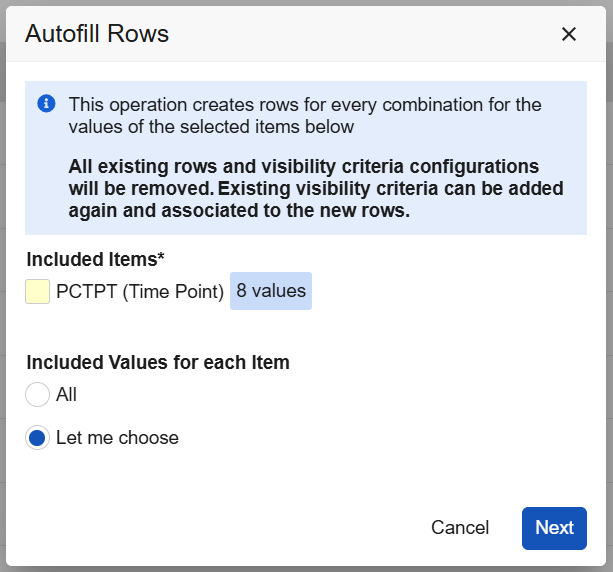
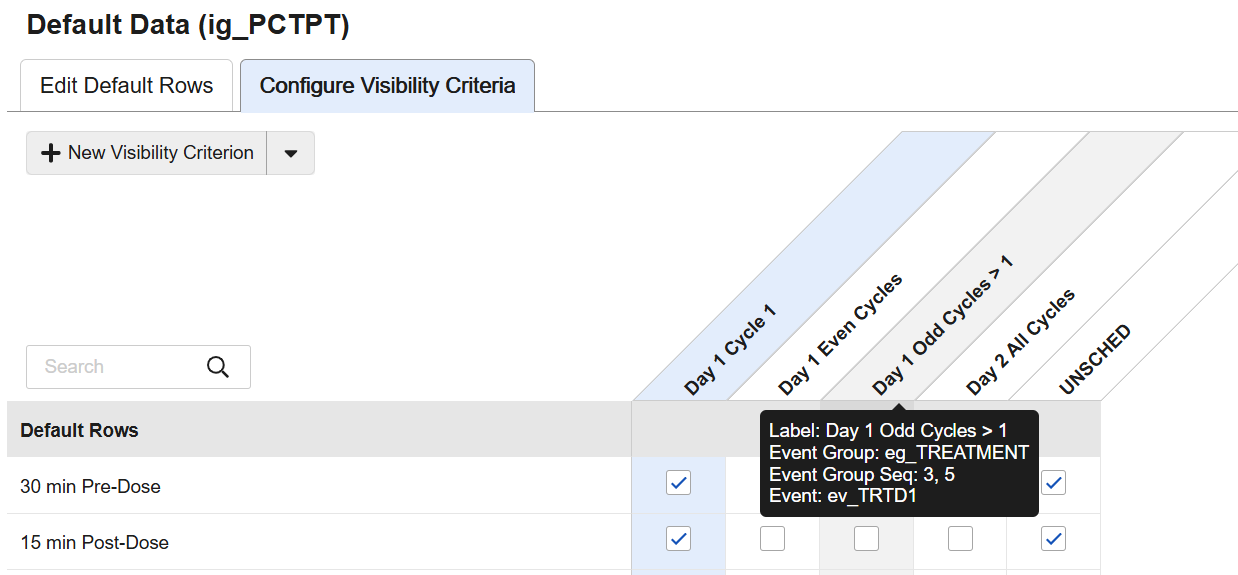
Studio: Configuration Improvements for Default Data and Visibility Criteria 25R2.3
Use Case
With this release, Studio provides better usability for default data and visibility criteria with UI improvements, new warnings, and better sorting when updating or adding defaults.
Description
When study designers change the default values for an item group and the changes impact the existing visibility criteria, Studio will now provide a warning in the following cases:
- Adding a row before a row that has a configured visibility criteria record
- Adding a new row at the top or before another row (new row, insert 1 row above, insert 1 row below)
- Dragging/dropping a row before a row with visibility criteria
- Removing a row before a row with visibility criteria
- Changing a row value
(Note: Adding a new row as the last row is not impacted and will retain the previous visibility selections.)
A new text warning will also be seen within the Autofill Rows dialog.
When configuring visibility criteria, the criteria will automatically sort alphabetically within the grid once the page is reloaded. New hoverover messaging on the visibility criteria provide the configuration details from directly within the grid.
Enablement & Configuration
These changes are automatically available in Studio.
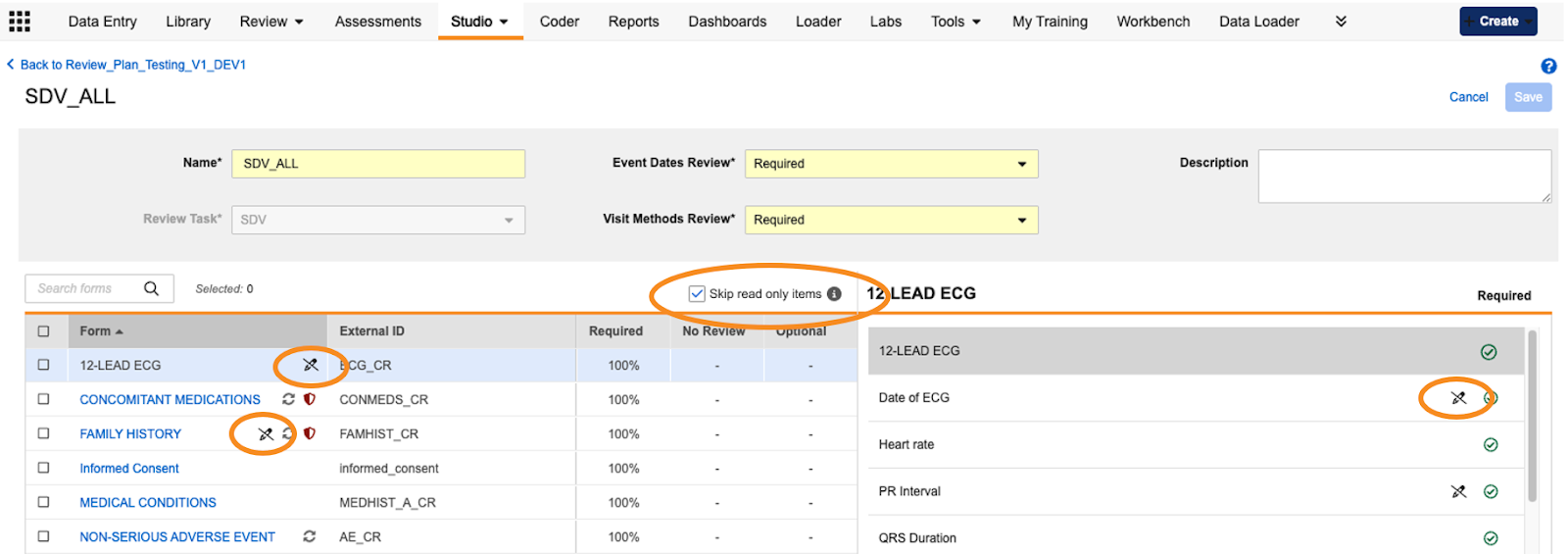
Read-only Items Default to No Review 25R2.2
Use Case
Providing options and setting the default state for handling Read-only Items allows study teams the flexibility to include or skip these items from being counted in SDV or DMR percentages within the Studio review plan configurations.
Description
When configuring review plans in Studio, read-only Items will be set to “No Review” by default in new plan configurations. A new checkbox option, “Skip read only items”, allows study designers to exclude the read-only Items from being updated when using bulk actions to revise review plan configurations. The checkbox is selected by default. A new read-only icon and hoverover text display next to the Forms and Items, visually indicating Items that are read-only and Forms containing read-only Items.
Enablement & Configuration
This feature is automatically available in Studio for the General Release. Read-only Items will be set to “No Review” in configurations following 25R3. Existing plans will not be impacted. The icon will appear for new plans configured following 25R3 and also for existing plans. Changes to review plans for existing studies require a versionless deployment or can be included with a new casebook version.
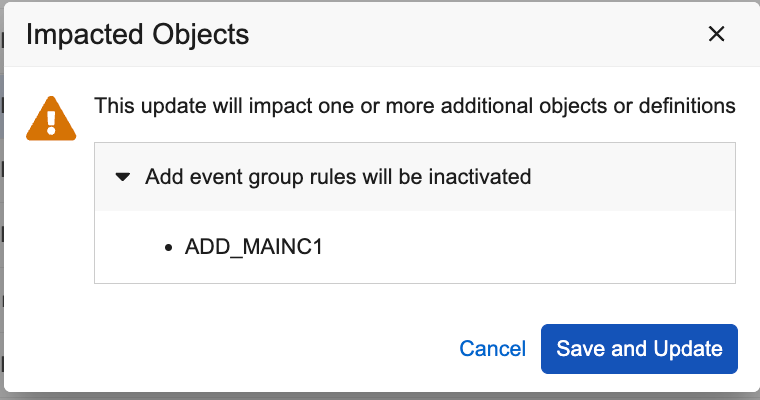
Automatically Inactivate Related Rules on Event Group Changes to Non-Dynamic 25R2.3
Use Case
Study designers sometimes need to change the properties of an Event Group, switching it from Dynamic to Unscheduled, or moving the group to be the first event in the schedule. By automatically inactivating rules and warning users as they make changes to dynamic settings for Event Groups, this change helps ensure more complete and accurate revisions to study designs.
Description
When you switch an Event Group’s properties from Dynamic to non-Dynamic, Studio automatically sets all related Add Event Group rules to Inactive. A warning will appear alerting the study designer of their intended actions and listing all the rules that were impacted.
Enablement & Configuration
Automatically available in Studio when revising Event Group properties from Dynamic to Non-Dynamic.
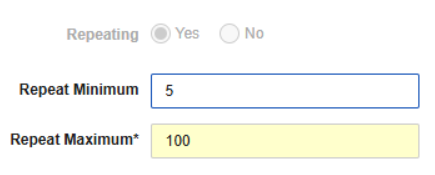
Minimum Number of Repeating Forms 25R2.3
Use Case
Adding a minimum number of repeating forms will automatically add form repeats to the study schedule, which helps Site users remember to enter the forms. With this feature, CRAs and Data Managers can also open queries on missing forms. This enhancement also provides better visibility and reporting metrics for repeating forms like AE or CM, when they are missing or incomplete.
Description
The Repeat Minimum field is now available in the form properties for repeating forms. Entering a minimum value (1-9999) will automatically create the specified instances of the repeating form within the schedule in Data Entry, removing the need to select the Add form button. Override values can be configured in cases where a separate minimum number of form repeats is required for specific event locations. In the Forms screen in Studio, the new Repeat Minimum column will display so that users can easily view the designs across the list forms.
Site Users
An Expand Repeating Items toggle switch will now display in data entry at the top of repeating forms which also contain repeating item groups.
Study Designers
The entered minimum repeating number is shown in the RMin column of the Schedule Tree tab in the SDS and also on the Annotated PDFs generated from Studio. When running the Studio Comparison (Diff) Report, the configured minimum number will also be evaluated for changes and documented in the Schedule and Detail tabs of the report.
When copying designs from libraries or other studies, the value for the Repeat Minimum will be included along with the Form Properties.
Dynamic forms with minimum repeats will still adhere to dynamic rules, and will only be available when the rule logic evaluates to true.
Designers can change the number of minimum repeats that will apply to new subjects and existing forms will not be marked for removal. Increasing the minimum number as part of a retrospective amendment will initiate the new forms within the schedule.
Additionally included with this features is an option in Studio where designers can restrict data entry users from adding new rows to the repeating item groups with the Disable New Rows property. The Disable New Rows property is available for new studies only. Existing studies will not see this option in the form properties. For new studies, this setting can be used for schedule driven default data on both repeating and non-repeating forms.
Extracts and Reports
The following columns have been added to the 25R3 Study Data Extracts (SDE) version:
- Form Definitions file
- Repeat Min
- Casebook Schedule file
- Event Group Repeat Max
- Form repeat Min
- Form Override Repeat Min
- Form Repeat Max
- Form Override Repeat Max
- Item Group Repeat Max
Missing and incomplete forms based on the Repeat Minimum will be included in the Standard Template: Detailed Missing Forms Report (V3), Form Progress, and Subject Progress Listings.
Enablement & Configuration
Immediately available within Studio configurations. The Expand Repeating Items option in Data Entry will display automatically but will remain off by default for existing studies and will default to on for new studies.
Set Obsolete on Dynamic Rules 25R2.3
Use Case
Users who had forgotten to re-run the Run Rules job after revising the rule Action or Action Scope for dynamic rules could have unexpectedly seen the dynamic Form, Event, or Event Group still present within the Casebook, or removed, or marked for removal based on the rule results from the previous rule configuration. A new backend status helps ensure expected results when dynamic rules are revised.
Description
Within the Admin area, Rule Results will now include an Obsolete Status to track prior executions of dynamic rules and later reevaluation of rules due to revised rule configurations. In particular this helps address dynamic rules where the dynamic action scope or the rule action (Add Form, Add Event, Add Event Group) is changed. With the new obsolete status, EDC will more accurately track and cleanup rule result records to ensure the processing of dynamic Forms, Events, and Event Groups is as expected.
In the Run Rules job output file, including the preview output, if an object is removed or marked for removal because its corresponding rule result became obsolete, the “Action Details” column will state “Obsolete Rule Result Processed”.
Enablement & Configuration
This feature is automatically available in EDC Tools when utilizing the Run Rules job.
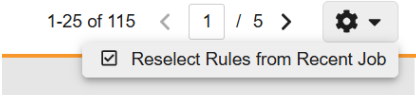
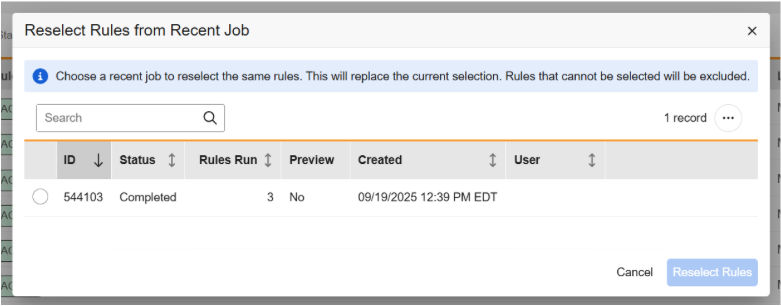
Reselect Rules from Recent Rule Job 25R2.3
Use Case
This feature adds a new menu option that allows users to select a previous rule job to reuse the same set of rules, saving time and effort.
Description
A new action menu option is available in the Rules grid which allows users to reselect rules from a recent job.
Selecting this option opens a new dialog that allows users to search and select the job from which to automate the rule selections. The rules from the recent job will be highlighted and users will be able to unselect rules or select additional rules before running the new rules job. Disabled rules will not be included in the current job selections.
Enablement & Configuration
This feature is automatically enabled.
Learn More
Update Rule Execution Order for Subject Status Rules 25R2.3
Use Case
Rules that include references @Casebook.subject_status__v in the logic, such as Override Review Plan rules, could potentially process prior to a Subject Status rule and miss the newly assigned subject status. This feature ensures the Subject Status rules are evaluated ahead of other rules to ensure the updated subject status.
Description
Rules that process from either a Form submission or when running the Run Rules job will be prioritized in the following order, moving the Set Subject Status rules ahead of other rules such as Review Plan Override and Query rules.
- Derivations
- Add Event Group
- Add Event
- Add Form
- Set Subject Status
- Other rule types
Enablement & Configuration
This feature is automatically available as data is saved or when running the rules job and the rules process.
Studio Copy Improvements 25R2.2
Use Case
Enhancements to Studio copy capabilities provide greater reuse across studies and vaults, with the ability to copy Imaging forms, a new Preview capability for study designers to ensure copy selection results, and improvements to the copy log.
Description
Copy Preview
A new Preview button appears in the Copy dialog when copying Event Groups, Events, Forms, Rules, and Codelist/Units. Clicking the button starts a preview job and an email notification with a download link to the copy log is sent to the user when the preview job completes. The filename is appended with “_P” to note the results are a preview.
Copy preview generates the same log as the copy operation but the definitions are not saved in the destination. This allows users who are unsure of which copy options are most suitable to understand how certain definitions may be processed in the destination.
When copy preview is selected, the copy window remains open with your selections retained so that you don’t have to reselect those values after you have reviewed the preview copy log.
Copy Imaging Forms
For vaults and Studies using Imaging, these forms can now be copied across studies within the same vault. When both the source and destination studies have Imaging enabled, the imaging Form(s) will be available to copy through the Form selection field. The system-provided Imaging Item Group, Items, and Expected Modality will not be duplicated during the copy operation. Imaging Form copy also respects other Form differences and uses the existing capabilities for the Use Existing or Make a copy, and Include Rules options. Skipped imaging exam Item Groups and Items along with noted differences will be included in the copy log.
Skipped Archived Rules Not Logged During Form Copy
When a source Form with archived (deleted) rules is copied, the archived rules are not logged.
System-managed Lab Items and Item Groups Matched by Name in Diff Report
When running a Studio Comparison (Diff) report with lab Forms included, system-managed lab Items and Item Groups are now matched based on the names rather than the system keys. The report will no longer show system-managed Items and Item Groups as differences.
Enablement & Configuration
These copy improvements are automatically available in Studio.
Studio Copy: Rule Identifier Matching 25R2.2
Use Case
When copying user defined rules, rule identifiers match and update with Studio configured object names to more successfully copy rules and reduce efforts for study designers.
Description
The system uses a number of system keys to uniquely identify study definitions when copying rules. This is because definition names can change from the first Study version that they are present in. Due to this behavior, it is not always clear why a definition or rule may not be copied.
When copying rules, the system will attempt to reconcile portions of the rule identifiers that had previously been shown as “null” by using the names of the Event Groups, Events, Forms, and Items found in the destination Study/Library. The name of the definition object will replace “null” when a matching name exists between the Studies/Library. As the rule loads, the system will first attempt an exact match on the system keys of the object to which the identifiers point. If the key does not match, then the system will attempt to match on the configured names of the objects. If no match is found between the Studies or Library, then the portions of the identifiers will show “null” and the status of the copied rule will be invalid.
The object name will be shown when the keys don’t match on copy in the following scenarios:
- If a definition of that type exists in the destination with the same name but a different key from the source Study definition.
- If you added a definition after the copy to the destination (either via a copy or manually) and it matches the name in the rule expression.
The null value will be shown when the keys don’t match on copy in the following scenario:
- When neither the name nor key match a definition of that type in the destination.
This change doesn’t apply to the Form or Event Group selected in the Rule Details panel or to the rule action identifier. If the keys for those objects do not match, those values will still reflect “null”. Name matching for these elements during copy operations will be evaluated as a future enhancement.
In the resulting log files, rules that could not be matched on names will be indicated in the log as “Rule copied and marked invalid - missing destination study references”.
Rules that match on names will not show as modified within the Studio Comparison Report (“Diff” report).
The Study Design Specifications (SDS) will also correspond to the updated rule expression, documenting the rule identifiers with the updated names.
Enablement & Configuration
This feature is automatically available in Studio.
New Column in Studio Diff Report for Comparison Rules V1 25R2.2
Use Case
A new column in the Studio Comparison (Diff) Report helps clarify the report records when V1 Comparison Rules are changed or archived. The Rule Name column indicates which rule was updated in cases where the Event Group, Event, Form, Item Group, Item Operator, and Compare To values are blank.
Description
A new column “Rule” has been added to the Comparison Rules tab in the Studio Comparison (Diff) Report. The new column will include the rule name where there is a difference between the study environments and helps clarify the difference in cases where the rule is reported with [not present] for the source study and [present] in the target environment. The Rule column helps indicate where modifications occurred in the following scenarios:
- The comparison rule is archived in the source study and unarchived in the target study
- The comparison rule exists in the target study but not the source study
- The comparison rule is present in the source and target studies, but the rule was updated in source study, resulting in separate rows to show the different rule
Enablement & Configuration
This feature is automatically available in Studio and is applicable for studies using Comparison Rules V1. This change is not applicable for studies using Comparison Rules V2.
Studio - Enable Configuration Settings for New Studies 25R2.2
Use Case
Specific study settings will now be automatically enabled when creating new studies,reducing efforts for study designers. The default settings and adoption of these features help sites and CRAs gain efficiencies with managing queries and ensure the study operates in the study language.
Description
New studies will have the Enforce Study Language, Enable Team Query Restrictions, and Enable Quick Queries settings enabled by default. Study Designers will still have the option to deselect these settings, if necessary. We encourage users to use the default settings to gain benefits from the associated Quick Queries and Team Query Restrictions features and to ensure the study language.
The settings for Query Team for System Queries, Study Language, and Study Locale will remain blank. Users will have to provide these settings as part of the study settings.
Enablement & Configuration
Automatically available for new studies starting after the general release. Note that settings from the source study will be used when copying a study.
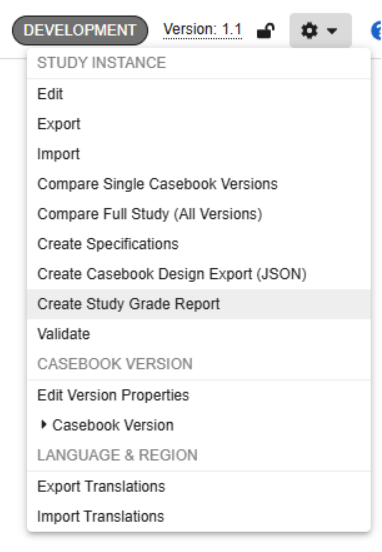
Study Grade Updates 25R2.3
Use Case
This feature includes Improvements to Study Grade and additional information within the report to further assist users with their designs and speed up review times, allowing designers to review report results ahead of the TST deployment.
Description
Users can run the Study Grade Report ad-hoc from a study’s DEV environment with the new Create Study Grade Report option in the studio action menu. Once the report completes, the user will receive an email notification with a link to the report.
The SDS will be included with Study Grade reports that are automatically included in the deployment to TST.
The codelist_display check has been updated for the following cases:
- The Threshold Lower Range has been updated to 4
- Repeating item groups and editable grid views are considered to log better results
- Read only and derived items are no longer included in the evaluation
Additional information is provided in the Object Names(s) column to help users locate and correct the exception.
A new column, Form, has been added to the Exception Log tab, which includes the name of the impacted form related to the following checks:
- form_unnecessary_copy
- codelist_consistency
- codelist_display
- rule_blank_checks
- rule_scope
- rule_dynamic_results
- rule_complexity
- rule_qualified_identifiers
- form_order (new)
Enablement & Configuration
Automatically available in Studio for users with the View Study Grade permission.
Learn More
Study Language Enhancements - Site Closeout Details & Site Closeout Activity Details 25R2.2
Use Case
To provide consistency and further enhancements for studies using the Study Language feature, specific text within the Closeout Activity screen has been updated to utilize the selected Study Language.
Description
When the study setting for Enforce Study Language is set to “Yes”, the Closeout Activity, located within the information icon in the Closeout Status column in EDC Tools > Sites, now displays the Events in the study’s selected Study Language.
Enablement & Configuration
Automatically available for new Closeout Activity events in studies enforcing study language. Studies that are not enforcing study language will continue to see the Closeout Activity in the user’s language.
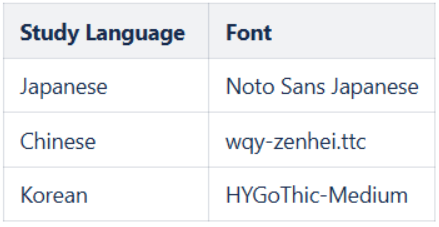
Updated Fonts for Chinese, Japanese, and Korean Languages in PDFs 25R2.2
Use Case
This feature corrects font inconsistencies in the Detail and Closeout PDFs for specific Chinese, Japanese, and Korean fonts in studies where the study or user has these languages selected.
Description
In studies where the Enforce Study Language setting is set to “Yes” and the selected study language is Chinese, Japanese, or Korean, or in studies where the Enforce Study Language setting is set to “No” and the user’s language is Chinese, Japanese, or Korean, the following fonts are ensured in the Detail and Closeout PDFs:
There may be specific cases in which a given language character is unavailable. In those cases, users can expect the following behavior:
- If the study language is Japanese and the character is not available in Japanese, the character will be seen in Chinese.
- If the study language is Chinese and the character is not available in Chinese, the character will be seen in Japanese.
- If the study language is Korean and the character is not available in Korean, the character will be seen in Chinese.
Enablement & Configuration
This feature is automatically available when generating the Detail and Closeout PDFs in these languages. Note that when Study Language is enforced, the font for the selected study language will display. When Study Language is not enforced, the fonts will be seen in the user’s language.
Study Administration
Features in this section apply to System Tools or EDC Tools, a study-level administration area for Veeva EDC.
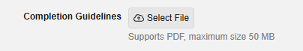
Completion Guidelines 25R2.3
Use Case
By accessing Completion Guidelines directly in EDC, site users can raise the quality and consistency of their data entry in EDC, reducing additional effort with questions and queries.
Description
Study designers in DEV/TST and study administrators in PROD, with the Edit Study Settings permission, will be able to upload, view and remove Completion Guidelines in EDC Tools > Study Settings. A new Completion Guidelines setting with a Select File button allows PDF files up to 50 MB to be uploaded. The file name has a 250 character limit.
Once an EDC Tools user deletes the PDF file, it is completely removed from the system. It is not retrievable from EDC and users will need to reload the new source file. Completion guidelines are not deployed. Files are uploaded uniquely to the applicable environments.
Once a file is uploaded, the file will be available from a new Completion Guidelines option at the top right of the screen in Data Entry and Review. Users with access to subject data will see and be able to select the link, which opens the file in a PDF Viewer.
Enablement & Configuration
This feature is automatically available for configuration in EDC Tools upon release. A study administrator must add completion guidelines in EDC Tools before those guidelines are visible to end users.
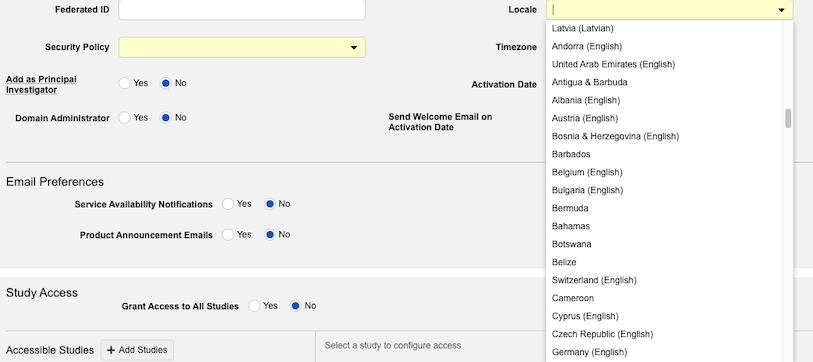
New Locales for Veeva Clinical Data Users 25R2.2
Use Case
This feature provides consistency with user locales added at the platform level.
Description
New user locales added at the platform level are now also available for Veeva Clinical Data users. User administrators will see an additional 213 locales added to the Locale dropdown menu in the user record.
Feature support for VeevaID users will be added following the 25R3 general release. In the interim, an error message will display when attempting to set up any VeevaID user in EDC with an unsupported locale.
Enablement & Configuration
This update is immediately available.
Learn More
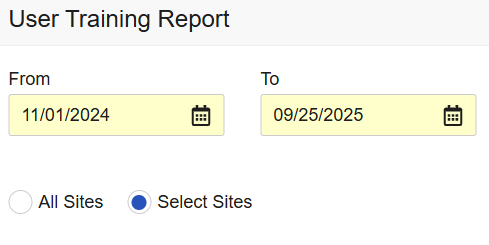
Training and User Access Report Enhancements 25R2.3
Use Case
These updates provide expanded access to the User Access Report and User Training Report.
Description
The following changes were made to expand access to these reports:
User Training Report: The User Training Report, accessible from EDC Tools, has been updated to allow users to run the report for All Sites or Selected Sites. Additionally, API endpoints have been created for the report.
User Access Report: We’ve removed the six-month window limit when running the User Access Report from System Tools. API endpoints have also been created for this report. Report duration limits are in place for jobs initiated by the API, with datetime values supported. The report duration must be between one hour and six months.
For more information on API endpoints, read the EDC API Developer Release Notes.
Enablement & Configuration
These updates are immediately available.
Learn More
Icons Added to Normal Range Indicator Values in Review Tab 25R2.2
Use Case
This update provides consistency in the display of lab normal range indicator values between Data Entry and Review.
Description
Lab Normal Range Indicator icons will now also be visible on lab forms in Data Review.
Enablement & Configuration
These icons will be immediately visible in the Review tab for applicable lab results.
Learn More
Disable Audit during PPT Study Deletion 25R2.2
Use Case
This update helps to streamline deletion records and prevent performance issues which could have been encountered in a TEST or PPT vault, where the PPT environment is located, and following the deletion of the PPT environment.
Description
Following the deletion of a PPT environment, the system does not need to retain specific deletion records as seen by Vault Owners within the Object Audit History in Admin and which occur from a previous PPT environment deletion or from copying the data into the PPT environment where that PPT environment is now deleted.
The deletion records listed here from the Object Audit History and which were previously retained as part of the PPT environment deletion are now cleaned up and removed from the Object Audit History records. Note, actions and revisions to data within the active PPT environments are always included in the audit trail.
- item2__v
- item_group2__v
- item_history2__v
- item__v
- item_group__v
- item_history__v
- query_message__v
- review_state__v
- execution_data_state__v
- coding_request__v
- rule_result__v
- signature_binding__v
- signature_readiness__v
Enablement & Configuration
This feature is automatically available for the General Release. This feature is not testable by study end users and is an adjustment only to the Object Audit History accessible in Admin by Vault Owners.
Deployment UI & Processing Improvements 25R2.2
Use Case
We’ve introduced enhancements to the study deployment process which includes UI improvements to help clarify and reduce deployment failures and reduce the need to contact Veeva Support to help successfully re-deploy design changes.
Description
During study deployment, the deployment package will process as a series of smaller transactions grouped by casebook version and by versionless portions of the study design. Successful and unsuccessful portions of the design will be tracked in the background as part of the deployment process. When the Delete Study Data or Generate the Detailed PDF options are selected as part of the deployment, those files will be processed prior to the design import to reduce deployment failures.
When a deployment fails, the appropriate transaction causing the failure will be noted in the log.
In the study environments page in Studio, UI updates help notify designers and testers when the environment is temporarily unavailable and that a re-deployment step is needed. A red error icon and a link to the Deployments page in help will display in the new hover over message.
In Studio, if an environment is temporarily unavailable, users will not be able to copy casebook definitions to or from other studies. Users will be prevented from selecting the environment when running Diff reports while it is unavailable.
In EDC Tools, while attempting a deploy action for an unavailable environment, the system will display the following error message: “This study has a casebook definition failed deployment and has been marked unavailable.” When attempting to restore another study design to an unavailable environment, the dropdown menu will not include this environment in the selection list. On the Sites page, setting the active version will not be allowed in the UI or through the import and a new dialog will display if the user attempts to run a Retrospective Amendment for the unavailable environment.
Additionally, as part of this feature, if a user attempts to alter the URL to navigate to a study environment, they will be automatically re-directed to the Study Master page for that study.
Note that this feature will not impact Production environments, as a successful deployment to TEST is first required before deploying the version to PROD. To make a TEST environment available again, the user can remove data that would be impacted by the design changes prior to attempting to re-deploy the new version.
Enablement & Configuration
This feature is automatically available for the General Release.
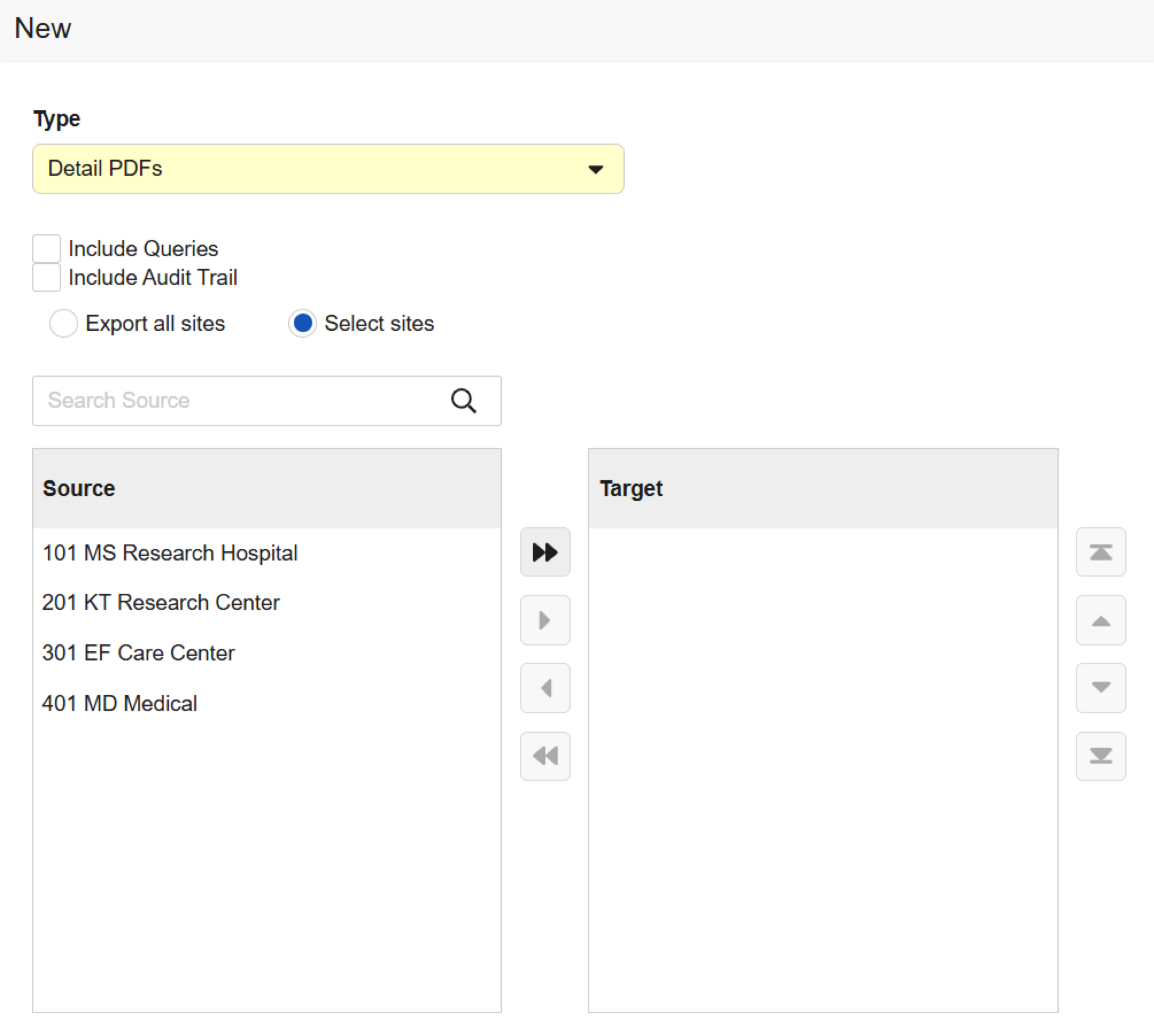
Job Site Selection Enhancement 25R2.2
Use Case
Improvements to the site selection within job dialogs provide a consistent experience and help Data Managers differentiate between sites. This is particularly useful in cases where two sites have different numbers but the same site name.
Description
The Source and Target site selections have been updated to include both the site name and site number when running these jobs. The search feature within the dialog now additionally supports searching on the site number. Jobs impacted by this update include the following:
- Detail PDF
- Core Listings (EDC Tools and Review tabs)
- SDV Re-assignment
- DMR Re-assignment
- Recalculate Normalized Datetime Values
The hoverover information has also been updated to include the site name along with the site number.
Enablement & Configuration
This feature is automatically available.
Pre-deployment Difference (Diff) Report 25R2.2
Use Case
In cases where study designers forget to generate the Studio Comparison Report prior to deployment, the system will now automatically generate the Diff report.
Description
When deploying study design changes, the “Diff” report (Comparison report) will automatically generate prior to the deployment import process. Included in the Deployment History files, the Excel formatted file will include the “_pre” suffix, after the date/time stamp. For example:
Diff_StudyName_TST2_3_to_StudyName_DEV1_3_073120251245_pre.xlsx
These files will generate regardless of whether the deployment succeeds or fails.
Enablement & Configuration
This feature is automatically available for the General Release.
Learn More
SDE - Support Lab Localization Timezone & Fasting Status in SYS Files 25R2.2
Use Case
These additions provide additional information from EDC in the SDE exports.
Description
The 25R3 version of the SDE includes the following updates to Lab system files:
- To support new data elements added in a previous release, we are including Lab Location Time Zone (as LABTIMEZONE) in the SYS_LABLOC file and Fasting Status (as LBFAST) in the SYS_LABRANGES file.
- We have renamed the column for Female Cycle to LBFEMALECYCLE in the new version of the SDE, located in the SYS_LABRANGES file.
Enablement & Configuration
These new columns are available when using the new version of the SDE.
Learn More
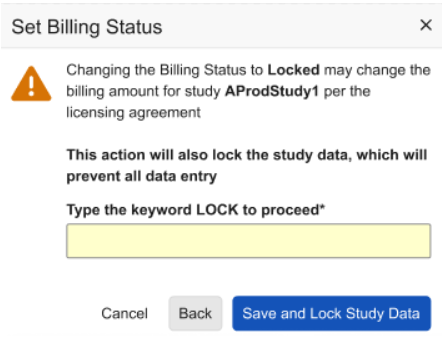
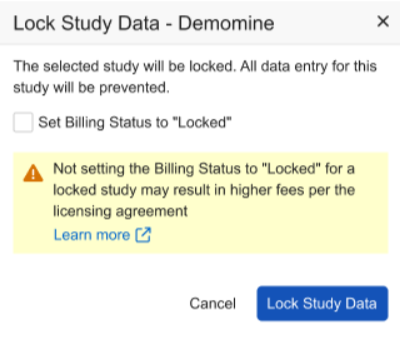
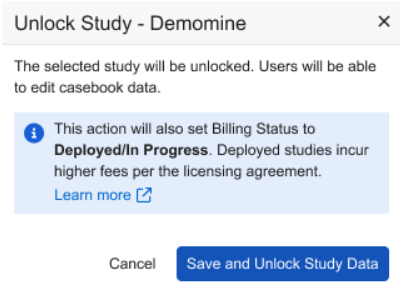
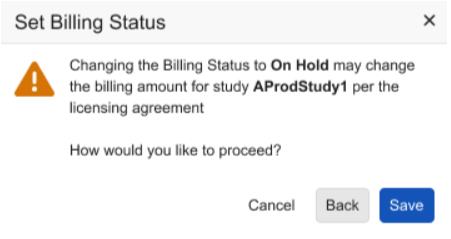
Study Locking & Billing Enhancements 25R2.2
Use Case
We’ve updated dialogs and confirmations that are seen when setting Billing Status and locking the study data to help study administrators better manage the sites from continuing to enter data and to help avoid additional invoicing.
Description
When selecting Study Data Lock, non-ELA customers will see an updated dialog with the following checkbox option, which will be automatically selected: Set Billing Status to Locked.
When locking the study data from the Billing Status action, the study administrator will be prompted to type the word LOCK to ensure confirmation of the Study Data Lock action.
When unselecting the checkbox, the user is presented with additional information and a link to the help pages.
When selecting Study Data Unlock, an updated dialog will notify users that the Billing Status will automatically be updated to Deployed/In Progress.
When setting the Billing status to On Hold, Deployed/In Progress, or Decommissioned dialogs provide informative text to notify study administrators of potential changes to billing based on the selection.
The Billing Status picklist label has been updated from Post-data Lock (This does not lock the study data) to Locked.
Enablement & Configuration
This feature is automatically available for the General Release. Only non-ELA customers.
Learn More
Timezone Label Sync 25R2.2
Use Case
Specific time zone labels have been updated to ensure consistency with Vault platform and prevent mismatches when configuring ClinOps (CTMS) to EDC Connections.
Description
We’ve updated timezone picklist labels for timezone__v, which is utilized for sites and lab locations, to match the platform (and CTMS) timezone labels for timezone__sys. The updated timezone labels are viewable in these screens and dialog picklists.
- New/Edit Study Sites dialog
- Sites grid (EDC Tools)
- Site import file
- New/Edit Lab Locations & Normal Ranges
- Lab Locations & Normal Ranges grid
- Import Lab Locations
Customers should update the timezones in their CSV files used to import EDC sites and lab locations. During the import of sites and lab locations, users may encounter an error in the import preview if the updated timezone labels are not used. Following the release, the CSV files can be exported and used for subsequent imports to ensure the import file will include the updated labels.
Enablement & Configuration
This feature is automatically available.
Learn More
Labs
Features in this section are new features for the Labs module of Veeva EDC.
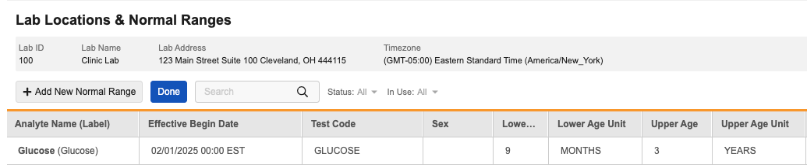
Age Range Enhancements for Normal Ranges 25R2.3
Use Case
This feature supports local lab normal ranges for pediatric studies, where lower and upper age limits may be based on different units (9 months to 3 years, for example). This feature also allows support for single age normal ranges, like, for example, a normal range which only applies to subjects that are 18 years old.
Description
The Age Unit field for Normal Ranges has been separated into two separate fields for Lower Age Unit and Upper Age Unit.
Both values for existing normal ranges at the time of release will be populated with the value currently captured for Age Unit, as long as there is a corresponding Lower/Upper Age value.
Lab Range Import
The Lab Normal Ranges import template now includes columns for Lower Age Unit and Upper Age Unit.
Study Data Extract
To accommodate this change, the SYS_LABRANGES dateset in the 25R3 Version of the SDE will reflect the new column structure, as seen in the screenshot below.
Older SDE versions will continue to include only the deprecated single Age Unit field. As Lab Ranges are added or edited, this data will be out of date. For this reason, we recommend always using the 25R3 version of the SDE for updated SYS_LABRANGES data.
Standard Report for Lab Reference Ranges
The standard report Standard Template: Lab Reference Ranges now includes the new lower and upper age unit fields. This change will be visible to all users with whom the standard report has been shared.
Enablement & Configuration
This feature is automatically enabled.
Learn More
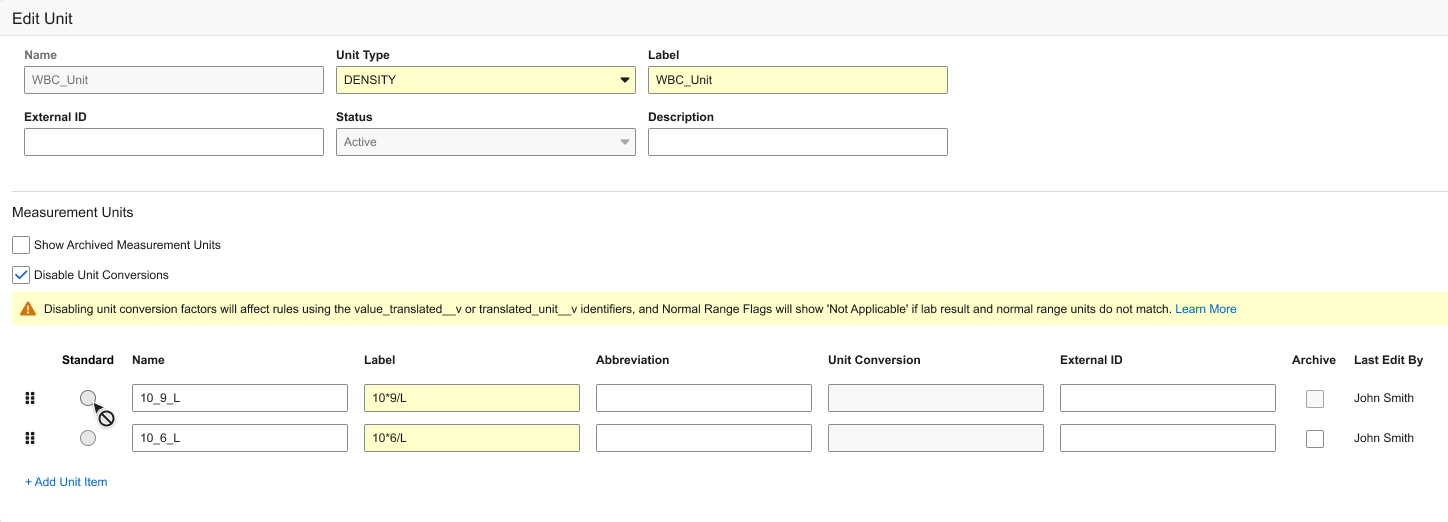
Allow Lab Unit Item Definition Conversion Factors to be Disabled 25R2.2
Use Case
This feature supports sponsors who manage all lab unit conversions outside of Veeva EDC, often for both local lab and non-EDC data. Disabling local lab unit conversion within Veeva EDC eliminates the need for reconciliation and other management of these conversions.
Description
When enabled for the vault, users creating or editing Lab Unit Definitions have the option to Disable Unit Conversions, which disables entry in the Standard and Unit Conversion fields.
A banner will display stating the following: “Disabling unit conversion factors will affect rules using the value_translated__v or translated_unit__v identifiers, and Normal Range Flags will show ‘Not Applicable’ if the lab result and normal range units do not match.”
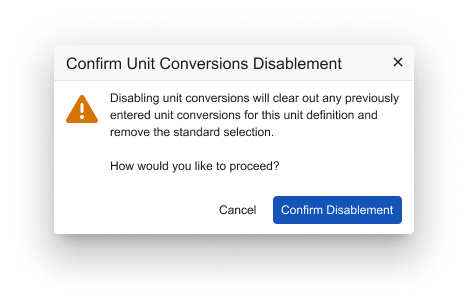
If there are previously entered conversions, the user will be presented with the following confirmation message: “Disabling Unit Conversions will clear out any previously entered unit conversions for this unit definition and remove the standard selection. How would you like to proceed?”
Upon confirming, previously entered conversions and Standard selections will be cleared out.
If a user later de-selects the Disable Unit Conversions option, the first unit item definition in the list will be marked as the standard (which can be edited but the user would need to enter new conversion factors).
Importing Lab Units
Vaults with this feature enabled can import lab units using a version of the import file that includes a column for the Disable Unit Conversion option. Users can download the template from the Units table within System General Settings. Note that users can only set this value when initially creating Lab Unit Definition records. Users can’t update the Disable Unit Conversion field for an existing record via the import and must update in the UI.
Vaults not enabled for this feature should continue using the template without this column.
Studio
If a study builder writes a rule that uses the value_translated__v or translated_unit__v identifiers for an analyte item not using unit conversions, the following warning will display: “The rule expression contains the value_translated__v or translated_unit__v identifier(s) for an Analyte which uses a unit definition that has conversions disabled. These identifiers will resolve to a null value and cause the rule to not evaluate as expected. Consider removing these identifiers from the rule.”
Normal Range Evaluation
If conversion factors are disabled for an analyte and the unit value entered by the site doesn’t match the normal range unit, the new Not Applicable icon will display with the following tooltip upon hover over: “When sponsors do not configure conversion factors, the system cannot calculate the flag if lab result and normal range units differ.”
Enablement & Configuration
Upon approval of the use case, this feature must be enabled by Support before it can be configured for any lab unit. In order to enable this feature, all studies in the vault using local labs must be using the global version.
Learn More
Analyte Library Source of Truth: Analyte Labels 25R2.3
Use Case
This update helps to ensure that the Analyte Library is the source of truth in all areas of Clinical Data, specifically for Analyte Labels.
Description
This feature updates the Item Property panel in Studio to remove the ability to configure the label and display override labels for all Lab Panel items to ensure that the analyte library is the source of truth in all areas of Clinical Data.
Studio, EDC, CDB and all other areas within Clinical Data, as well as the SDS, CDE, and SDE files will use the label as specified for the referenced analyte in the analyte library.
This feature will be automatically enabled for customers who don’t have any mismatches between analyte library labels and Studio analyte label item values. For customers that do have one or more mismatches, the feature will remain off, and Veeva will contact you to discuss resolution prior to enabling the feature.
Custom Report Impact
Item definition reports that include item labels will be impacted by this update. Item labels for LBTEST_ items will no longer be stamped on the item definitions and will no longer be updated in these reports once this feature is enabled. Because of this, we recommend using the Analyte Definition object instead of the Item Definition to get these values in a report.
Enablement & Configuration
This feature will be automatically enabled for customers with vaults that don’t contain mismatches between analyte labels and Studio Item Properties.
Learn More
Analyte Library Source of Truth: Analyte Precision and Length 25R2.3
Use Case
This update ensures consistency and clarity for precision and length values of local lab items per the configuration in the Analyte Library.
Description
The precision and length fields are no longer included in the item properties panel in Studio for studies using Global version of Local Labs, as these values are managed in the analyte library.
The Casebook Design Export (CDE) has also been updated to reference precision and length from the analyte library values.
Default Lengths We have updated the default length of the following lab-related items upon creation, to minimize the need for study designers to individually update them. Users can still modify these length values in Studio if needed.
- LBFAST Default Length: 100
- LBFEMALECYCLE Default Length: 100
- LBSEX Default Length: 100
The default length of analytes with data type codelist or text has been changed from 1500 to 100.
Custom Report Impact
After the release, precision and length values will no longer be stored on the item definition for Lab Result, Normal Range, and Normal Override items. Because of this, we recommend using the Analyte Definition object instead of the Item Definition to get these values in a report.
Enablement & Configuration
These updates are automatically enabled for all studies using the Global version of Local Labs.
Learn More
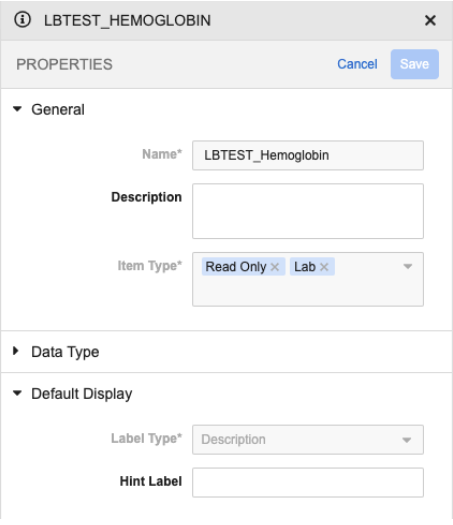
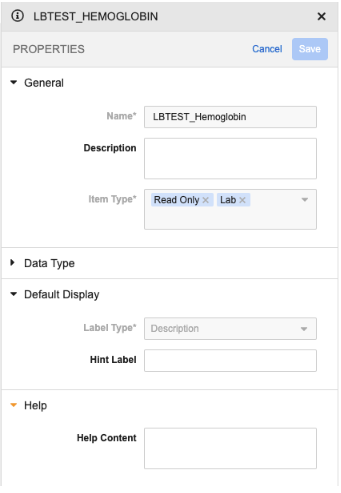
Local Lab Item Property Panel Updates 25R2.3
Use Case
This feature further supports the Analyte Library as the source of truth for lab panel items.
Description
We’ve added the ability to configure Help Content and Hint Label for LBTEST_ Lab Panel items (the analyte label), which will display in both Data Entry and Review, to allow study builders to provide supplemental instruction or information to users about the analyte.
We’ve removed the ability to configure Help Content and Hint Label for Lab Panel items other than LBTEST, as they are not supported in the Data Entry Grid UI for non-label items.
For codelist lab items (Lab Result, Normal Value, and Normal Value Override), the control type option is disabled and the Picklist option will be used.
Enablement & Configuration
These updates are immediately available.
Clinical DataBase (CDB) & EDC Clinical Reporting
The following are new features for the Veeva CDB application, EDC Clinical Reporting (the Veeva Clinical Data solution for data cleaning and reporting), or both.
Availability: Clinical DataBase (CDB) is only available to CDB license holders. Contact your Veeva Services representative for details.
Smart Reprocess of 3PD Packages 25R2.3
Use Case
Unnecessary reprocessing of third-party data (3PD) wastes system resources, resulting in longer batch processing times.
Description
The daily reprocessing of third-party data in production studies will now first check whether the last reprocess included D-002, D-003, or D-004 warnings. These warnings alert users when sites, subjects, and events (respectively) exist in the third-party data package but are missing from the primary source.
Reprocessing allows the data to load after the primary source has been updated to include the new sites, subjects, or events. If there are no warnings, there’s no need to reprocess the package, saving resources.
Enablement & Configuration
This feature is automatically available with the release. Note that only production studies have the automatic reprocessing of third party data.
Learn More
Restrict 3PD as Primary Source for Veeva EDC Studies 25R2.3
Use Case
This update ensures that third-party data providers cannot erroneously mark their data as primary for a study where Veeva EDC is used to collect data.
Description
A new validation ensures that a study designed to import data from Veeva EDC will not allow the import of third-party data as the primary source.
Enablement & Configuration
This feature is automatically available with the release.
Dynamically Include Column Values in Query & Observation Messages 25R2.3
Use Case
When writing queries or observations, especially in bulk, within a listing defined query, or through a check, tokens representing data from the listing row can be incorporated into the message. This allows the user to dynamically insert the data value into the message, to better customize the message to the site, or include important context in the observation.
This feature allows data managers to create many unique, data-rich queries in a single batch operation. For checks, this allows the study to automatically open queries with specific, relevant data points included in the message.
Description
CDB Workbench now allows data item values from a listing to be automatically included in a query or observation message, allowing for better customization of messages by including data from the listing.
Enablement & Configuration
This feature is automatically available upon the release.
Filter by Observation Status in Review Listings 25R2.3
Use Case
Observations written on data in a review listing can now be used to filter the listing to only those rows, based on observation status.
Description
New options in review listings allow for the filtering of rows based on the observation status (All, Open, Pending, Closed, None).
Enablement & Configuration
This feature is automatically available upon the release.
Listing Defined Quick Queries 25R2.3
Use Case
When a listing has a clear Objective, a Listing Defined query option can be configured promoting reuse and standardization across multiple Data Managers. Data managers still have the option to create a custom message, or use the Confirm Value quick query. With the new listing defined query feature, study teams can provide a way to set the language for a common query for each listing in the study, improving efficiency and comprehension for site responses.
Description
Review listings and Public custom listings will now allow a new quick query type to be defined to standardize query messages generated from the listing.
This option is available in the new Queries area of the listing’s Properties dialog. Once configured, a Listing Defined option is available for query type when opening a query.
Enablement & Configuration
This feature is automatically available to studies with Quick Queries enabled upon the release.
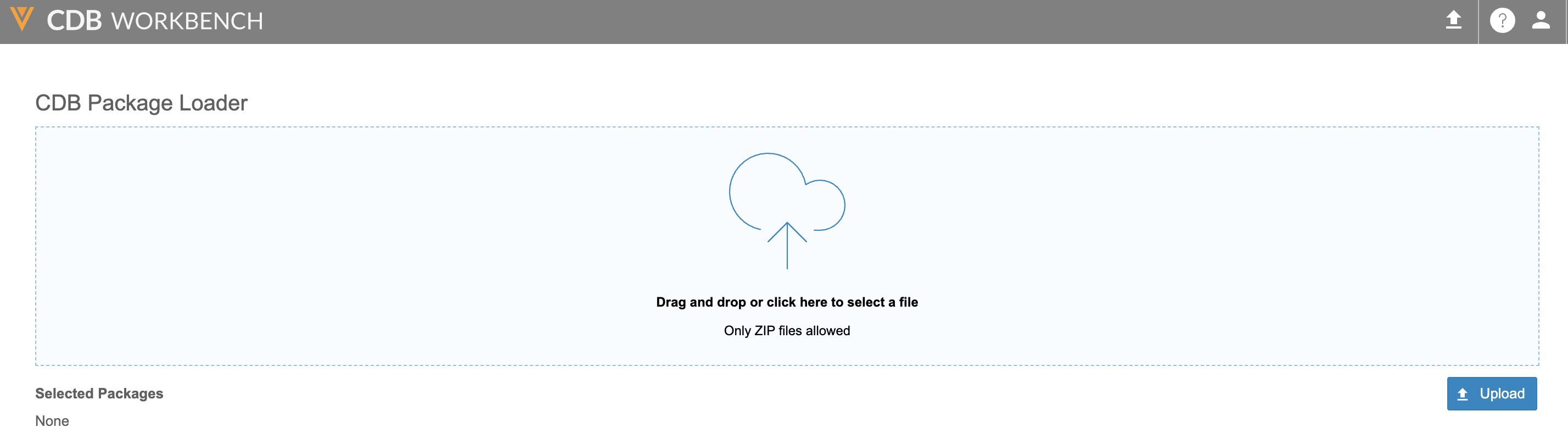
Drag-and-Drop 3PD Package Upload 25R2.3
Use Case
This feature provides a simplified interface for sending third-party and OpenEDC data to CDB Workbench.
Description
An option in the Workbench top navigation bar will allow users with the new Upload 3rd Party Data Packages permission to drag and drop ZIP files from their computer to upload data to Workbench.
The standard Data Manager, Lead Data Manager, CDB Data Provider, and Super User roles will be given the new permission.
Enablement & Configuration
This feature is automatically available upon the release.
Learn More
CDB Protocol Deviation Management 25R2.3
Use Case
Protocol Deviation creation in CDB Workbench allows for deviations to be created, for example, based on third party data, automating creation by comparing entered data to reference data (such as a prohibited medication list), and in batch from a listing.
Data managers can now create PDs while viewing data in CDB, and view deviations from within a listing using the listing decorations. Study teams can reduce manual work by configuring PD checks that automatically create and inactivate deviations as data issues are identified and resolved.
Description
Protocol Deviations (PDs) can now be created in CDB Workbench for a study. All PDs created on Veeva EDC data (manual and automated) will be visible in the Review Protocol Deviations page in EDC. Edits in CDB are restricted to setting a PD to inactive.
PDs can be created on EDC and third party data, at the Subject, Event, Form, or Item level.
New PD core listings are available: an all PDs listing, and a dedicated listing for each source. These core listings are available for download and inclusion in extracts and SFF.
The PD CQL syntax has been updated to include the following new attributes:
- Origin System
- Origin Name
- Origin ID
- Source
Enablement & Configuration
This feature is automatically available for studies enabled for Protocol Deviation management in EDC Studio.
Support Testing SFF in TST Studies 25R2.3
Use Case
This feature allows CDB Workbench studies to test SFF extracts in the TST environment.
Description
Test study environments now have the option to test the Study File Format (SFF) API. Once SFF is enabled for testing, it becomes disabled under two conditions:
- If 30 days pass since the last package download
- If the user never downloads the package
Users can re-enable SFF, but this requires a new, full export package to be generated.
Enablement & Configuration
This feature is automatically available to CDB Workbench studies after the release.
Learn More
CDB Workbench: System Listing Updates 25R2.3
Use Case
Including these ids allows customers to use the GUIDs for deep linking and more advanced joins to other Veeva provided data sets in downstream systems.
Description
This release added system globally unique ids (GUID) to system (SYS) listings to better align the CDB SYS listings with the SDE (study data extract).
SYS_SitesaddedSite.IDSYS_SubjectsaddedSubject.IDSYS_EventsaddedEvent.IDSYS_FormsaddedForm.IDSYS_LinksaddedForm.IDSYS_PDaddedPD.PID
SFF naming is SYSID for all fields except in “SYS_LINKS.csv”, where it is FORMID.
SYS listings included in data exports (including SFF) will automatically be updated upon the release, and will not display change detection.
With this release, we have also added @QRY.OpenToClose in the query listings and CQL. Changes to existing export definitions will display change detection decorations for impacted exports.
Enablement & Configuration
This feature is automatically available to CDB Workbench studies after the release.
Reference Data Import: Increase the Maximum Number of Columns to 35 25R2.2
Use Case
Reference data is imported into a CDB study to allow for custom listings to reference things like excluded medication lists or always Serious Adverse Event terms in the listing. This enhancement allows for more item data to be included in the reference data import.
Description
Imported reference data can now include up to 35 columns.
Enablement & Configuration
This feature is automatically available upon release.
Role Management & Security
Features in this section are enhancements to the System Tools > Role Management and System Tools > Users areas, as well as changes to standard Study Roles, security, and access control in Veeva Clinical Data.
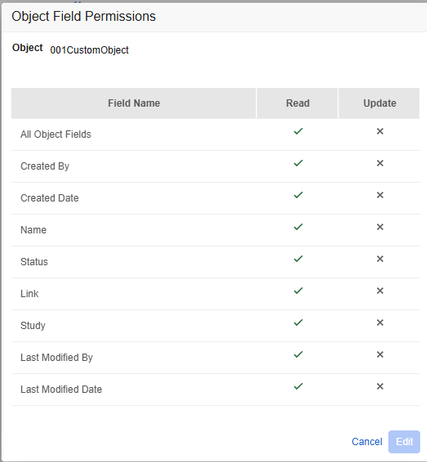
User Defined Permission Set Behavior Matches Admin UI 25R2.2
Use Case
This change makes the UI for permissions easier for users to interpret.
Description
With this release, the behavior of the Object Field Permissions Dialog for User Defined Permission Sets in System Tools is updated to match the UI experience in Admin, where the “All Object Fields” checkbox represents the total value state of all individual permissions underneath.
Enablement & Configuration
This update is immediately available.
Connections & Integrations
Features in this section are new connections or integrations with Veeva Clinical Data or enhancements to existing ones.
Clinical Operations - EDC Connection: Enforcing Unique Study Names 25R2.3
Use Case
The Clinical Operations - EDC Connection transfers the study name from Veeva CTMS to EDC. While the study is a single concept in CTMS, it appears in different instances with varying labels in EDC (e.g., “MyStudy_DEV” and “MyStudy_TST”). In a scenario where a CTMS Sandbox is connected to an EDC TST instance, the EDC production study name might be transferred via the Clinical Operations - EDC Connection to the EDC TST instance. This could overwrite the correct EDC TST study name if not previously adjusted in the CTMS Sandbox. Such incorrect naming can cause conflicts with further study deployment in EDC. With this release, you will no longer be able to rename a study in EDC if the CTMS study name matches the study name in any other EDC environment. This will particularly prevent issues when deploying studies to EDC production environments after testing the Clinical Operations - EDC Connection in TST environments.
Description
If the study name is transferred via the Clinical Operations - EDC Connection, renaming will fail, and a user exception message will be created if the study name from CTMS matches the study name of any other EDC instance. For example, if a CTMS Sandbox is connected to an EDC TST environment, the study name in CTMS will have to be adjusted from the production study name (e.g., “MyStudy”) to a different study name appropriate for the EDC TST instance (e.g., “MyStudy_TST”) for a successful integration.
Enablement & Configuration
Automatically enabled for studies using the Clinical Operations - EDC Connection. This functionality can’t be tested exclusively in EDC, but requires integrated testing with Veeva CTMS.
Learn More
Clinical Operations - EDC Connection: Display CTMS Link in EDC 25R2.3
Use Case
CRAs can now seamlessly navigate between EDC and CTMS systems using separate browser tabs, maintaining their logins while simultaneously checking records in both applications. This helps reduce the effort of logging in to multiple products and allows CRAs to more efficiently manage data in both EDC and CTMS.
Description
Studies using the Clinical Operations - EDC Connection will have a link in the top-right corner of both the Sites and Subjects view screens within the Review tab.
An info icon next to the link will show the name of the CTMS Vault when a user hovers over it. Hovering over the link will show the CTMS Vault’s URL.
Users with access to both systems can now navigate directly between CTMS and EDC using this new link. The link will navigate the CRA from a Site they’re viewing in EDC to the corresponding Site page in CTMS.
After clicking the EDC to CTMS Link, the system responds as follows:
- If a user has a previously active CTMS session, the new tab won’t require them to log in again.
- If a user has not signed into CTMS recently, they’ll be prompted to log in after clicking the link.
- If a user doesn’t have access to CTMS, they will be taken back to the CDMS Vault.
- If a user doesn’t have access to the site in CTMS, a Page Not Found error will appear.
Enablement & Configuration
Automatically available for new and existing studies that have the Clinical Operations - EDC Connection enabled.
Learn More
Safety-EDC Connection: Allow Unknowns and Time for Date of Death 25R2.3
Use Case
The Date of Death in Veeva Safety allows for full or partial date, including the use of unknown date parts, and the specification of date and time. This release enhances Safety-EDC Connection to account for this beyond the transfer of a full date.
Description
In Studio > Form Configurations > In Case of Death, the Date of Death field will now allow the mapping of date and datetime items with unknown date parts. Those unknown date parts will also be transferred via the connection.
Enablement & Configuration
This feature is immediately available for configuration for studies using the Safety-EDC Connection.
Safety Integration: Derivation Rule Execution to Mark Safety Case for Follow-Up Inspection 25R2.3
Use Case
Changes to the content of a Safety Case will mark the subject for inspection for a Follow-Up message to the safety system. Besides actual data change, this includes changes like linking or change of medical coding. With this release, any cross-form derivation rules targeting an item on a form being part of the Safety Case will result in an inspection of the subject for a Follow-Up message to the safety system.
This feature helps ensure that any data changes on the form are tracked for follow-up.
Description
If a cross-form derivation rule is executed that is targeting a derived item on a form in the Safety Case, the subject will now be marked for follow-up inspection.
Enablement & Configuration
This feature is automatically enabled for studies using the Safety-EDC Connection.
Safety-EDC Connection: Dynamic Vocabulary Synchronization for Concomitant Medication Drug Role 25R2.3
Use Case
The previously static picklist for Concomitant Medication Drug Role on the Safety Case Initiation Event safety form type is now dynamically retrievable from Veeva Safety.
Description
In System Tools > Connections > Safety, when running the Scan Vault Safety job on the Safety-EDC connection to synchronize EDC with the Veeva Safety vocabulary, the Value Translations for the Drug Role on the Safety Case Initiation Event safety form type (AE_CM_LINK_DRUG_ROLE) are now also synchronized.
For existing Connections and Studies, an administrator must re-run the vocabulary-specific Scan Vault Safety job to show the available Veeva Safety options for further mapping and deployment in EDC.
Enablement & Configuration
This feature is immediately available to be actioned in studies using the Safety-EDC Connection.
Safety Integration: Enhanced Date Matching for Inclusion Rules with Unknowns in the Event Start Date 25R2.3
Use Case
To determine the inclusion of additional safety-relevant subject information into a Safety Case, duration rules based on date matching with the start and end date of the Safety Case initiating event can be configured.
Prior to this release, a Safety Case Initiation Event start date (e.g. the serious adverse event start date) with unknown day or month would result in the exclusion of related form data where the duration rule comparison with the start and/or end date of the subjects’ additional safety-relevant information was ambiguous.
Now, an unknown day or month of the start date of a Safety Case initiating event (e.g. a serious adverse event) is handled with a positive bias to include additional safety-relevant information due to the configured duration rules. This means, if the unknown day or month does not allow to unambiguously judge if the related information should be included or excluded, it will be included, as long as it is not excluded due to other applying rules or settings.
Additionally for the study drug information, also the inclusion of multiple dispenses on the same day are now considered. Prior to this release, if more than one dispense occurred on the same day, the study drug information of only one dispense was selected for the Safety Case transfer. Now, all study drug dispenses on the same day that would meet the same inclusion criterion are being included in the send.
Description
In Studio > Form Configurations > Inclusion Criteria, date comparison rules can be configured to include additional safety-relevant information based on date matching with the start and end date of the Safety Case initiating event. The improved date matching applies to the inclusion criteria “All From Event Start to End Date” and “Days On/Before” resp. “Days On/After” the event start date for the Safety Case Initiation Event, Study Drug, Concomitant Medication, Medical History, Drug History, Test Results safety form types, and additionally “First Dispense”, “Closest On/Before” and “Closest On/After” for the Study Drug safety data type.
For example, if the Safety Case Initiation Event start date is July ??, a “First Dispense” on July 5th would now be included, which was previously excluded from transfer. If there were additional Study Drug dispenses on July 9th and 14th, all of these would be included due to the “Closest On/Before” rule, whereas none was transferred prior to this release. If only the year would be known as 2025, any information with a start date in 2025 would be included in the Safety Case when choosing the “Closest On/Before” option.
For the improved transfer of study drug dispenses that occurred on the same day, for a Safety Case Initiation Event Start Date of July 27th and the following Study Drug Dispenses (Start Date = End Date, i.e. end date is not mapped):
- July 15th (Form sequence #1)
- July 15th (Form sequence #2)
- July 20th (Form sequence #3)
- July 22th (Form sequence #4)
- July 26th (Form sequence #5)
- July 26th (Form sequence #6)
If the Inclusion Rule is configured to include “First dispense”, now both #1 and #2 are included.
If the Inclusion Rule is configured to include “Closest on/before”, now both #5 and #6 are included.
Enablement & Configuration
This feature is automatically enabled for studies using the E2BLink or Safety-EDC Connection.
Note: For live studies, the improved date matching might result in follow-up safety messages for existing Safety Cases.
Safety Integration: Enhanced Inclusion Rules for Medical History Forms without an End Date 25R2.3
Use Case
Medical History forms might not record an end date, or if recorded, not map it for transfer to the safety system. Instead, it’s mapped as Continuing and used for ongoing reported issues. Prior to this release, even if the Medical History record was marked as continuing, the missing mapping of a Medical History end date would result in a validation finding and the exclusion of the Medical History information when evaluated by date matching inclusion rules. Now, Medical History forms without a mapped End Date, but marked as Continuing, will be compared in the date comparison with an infinite end date.
Description
In Studio > Form Configurations > Inclusion Rules, date comparison rules can be configured to include medical history information based on date matching with the start and end date of the Safety Case initiating event. If the Medical History forms without a mapped End Date but a mapped Continuing that evaluates to true will be compared, the medical history End Date will be assumed as infinite. For example, if all Medical History information that occurred up to 365 days prior to the Safety Case Initiation Event start date is to be included in a case, now also records that started in this time frame and are recorded as continuing are included, as long as the medical history end date has not been mapped.
Enablement & Configuration
This feature is automatically enabled for studies using the E2BLink or Safety-EDC Connection.
Safety-EDC Connection: Extended Support for Custom Safety Text Fields 25R2.3
Use Case
In Veeva Safety, additional non-standard safety-related EDC data might be required for the Safety Case review. To support the flow of large text and accumulated EDC information to Veeva Safety, items inside a repeating item group can now be always mapped to Custom Safety Fields of the Text type, and EDC will automatically concatenate repeats.
Description
If an EDC item inside a repeating item group is mapped to a Custom Safety Field of type Text, the values of the repeating EDC items are concatenated. The values of each item will be separated by space and the concatenation order is based on the item group Sequence Number, in ascending order. At a limit of 32,000 characters the concatenated text will be truncated without further warning. The concatenated values are transferred as text to the mapped Veeva Safety Custom Safety Field.
For this, now also the selection of Form for Each Entry In allows the mapping of EDC items inside a repeating item group to a Custom Safety Field of type Text.
Enablement & Configuration
This feature is immediately available for studies using the Safety-EDC Connection.
Safety-EDC Connection: Extended Data Synchronization Period 25R2.3
Use Case
The Safety-EDC Connection transfers not only Safety Case data, but also all other safety-relevant subject information not deemed “in case” to be available for review in Veeva Safety. Once a Safety Case has been reviewed, there is no need to continually update all additional safety-related subject data through the connection indefinitely. Therefore, a limit of days after all events have ended for a subject needs to be configured for which the additional subject data is updated. This maximum period has now been expanded from 30 days to 500 days. Note that this setting does not influence updates to Safety Case data, where changes will trigger Follow-up messages indefinitely.
Description
In Tools > Safety Integrations > Safety Settings, the Data Synchronization Period for continuing updates to the safety-relevant subject data, which is not part of the actual Safety Case has been extended from 30 to a maximum of 500 days. The default remains 7 days.
Enablement & Configuration
This feature is immediately available for configuration in studies using the Safety-EDC Connection.
Safety Integration: Follow-Up Scan Job Enhancements 25R2.3
Use Case
The reliable processing of Safety Follow-Up Scans is critical during study conduct. To account for this need, we added more robust issue detection and failure notifications for the Safety Follow-Up Scan job.
Description
For any failed Safety Follow-Up Scan job, Vault will now send an email notification to the job owner and all email addresses or distribution lists defined in the Notification Emails field on the Connection.
Additionally, if the Safety Follow-Up Scan job owner has no longer permission to run the job, the job will be aborted with a failure message in the Job Log found in the Job History listing, and an email alert will be sent as described above.
To run the Safety Follow-Up Scan job, a user must be active, have study access, have access to all sites in the study, and a role that grants the Manage Safety Integrations and Manage Jobs permissions.
Enablement & Configuration
This feature is automatically enabled for studies using the Safety-EDC Connection or E2BLink.
Safety-EDC Connection: Linking Parent/Child Subject Information to Relevant Cases 25R2.3
Use Case
If the child of a trial participant experiences a serious adverse event in an unexpected pregnancy scenario, the site will create a separate Casebook and Safety Case for the child. The separate Casebook for the child will include demographic information, which links the child’s Safety Case to the parent Casebook. The current enhancement will additionally create a unique Case Link between the Safety Case of the child casebook and the relevant parental pregnancy or adverse event Safety Case in Veeva Safety. This will preserve the parent-child Case Link even if the parental subject number might be altered due to editing or deletion.
Description
By supplying the parental subject number in the child’s casebook, Veeva EDC will now additionally transfer the Parent Subject Global ID (parent_subject_global_id) with the case message to both identify a case as a child Safety Case and create a unique Case Link to the parent unexpected pregnancy Safety Case or most recent parent adverse event in Veeva Safety.
This functionality has no impact on the EDC user experience and can’t be tested exclusively in EDC, but requires integrated testing with Veeva Safety. The correct Case Link of the parent unexpected pregnancy and child Safety Cases must be traced in Veeva Safety.
Enablement & Configuration
This feature is automatically enabled for studies using the Safety-EDC Connection.
E2BLink: Multiple "Safety Case Initiation Event" Forms per Study 25R2.3
Use Case
Having more than one EDC Form that is required to trigger a safety case transfer from EDC to the safety system is a common scenario. For example, a study may have variations of the adverse event (AE) form by study drug, or the study may have a form for tracking unexpected pregnancy.
Prior to this release, E2BLink only allowed for the configuration of a single Safety Case Initiation Event, which is the form that triggers the safety case transfer. This resulted in a need to design a single comprehensive AE form to cover all potential cases.
With this release, also when using the E2BLink multiple Forms *can be mapped to the *Safety Case Initiation Event form type per study to initiate a data transfer. Configuring multiple EDC forms to initiate the transfer of a Safety Case allows for increased flexibility in AE form design in studies and libraries. It will also cover the key use case of collecting data for an unexpected pregnancy experienced by a trial participant on a separate form that will initiate a Safety Case independent of the AE form.
Description
In Studio > Safety Integration > Form Configuration, users are now able to create more than one Form Configuration using the Safety Case Initiation Event form type. Users can still only map one EDC Form to one Form Type per Study, for example, once a Drug Dispense Form is mapped to a form configuration, it can’t be mapped to another.
To account for the Safety Case Initiation Event optionality, we added an Inclusion Criteria section to the Safety Form Types listed below. This new Safety Case Initiation Event Which Includes selection allows the configuration of inclusion of the Form into the Safety Case if either any or only a selected Safety Case Initiation Event initiates a data transfer to the safety system.
- Concomitant Medications
- Drug History
- Medical History
- Study Drug
- Test Results
The configuration will default to Any. This additional configuration will not result in follow-up data transfers for existing Safety Cases while it remains unchanged.
As part of this feature, the following E2B fields are moved to the Safety Case Initiation Event form type. This move of the configuration will not result in follow-up data transfers for existing Safety Cases while it remains unchanged:
From the Concomitant Medication form type, the following E2B fields are now on the Safety Case Initiation Event form type:
- G.k.1 - Characterization of Drug Role
- G.k.8 - Action(s) Taken with Drug
- G.k.9.i.2.r.3 - Result of Assessment
- G.k.9.i.4 - Did Reaction Recur on Re-administration?
From the Study Drug form type, the following E2B fields are now on the Safety Case Initiation Event form type:
- G.k.8 - Action(s) Taken with Drug
- G.k.9.i.2.r.3 - Result of Assessment
- G.k.9.i.4 - Did Reaction Recur on Re-administration
The table below describes these fields:
| E2B ID | E2B Element | Information | Multiple Allowed? | Required Selection |
|---|---|---|---|---|
| G.k.8 | Action(s) Taken with Drug (Study Drug) | For each study drug. Multi-event case: Evaluation by Action Taken with Study Drug Behavior study setting. | Yes | Study Drug |
| G.k.9.i.2.r.3 | Result of Assessment (Study Drug) | For each study drug and related event | Yes | Study Drug |
| G.k.9.i.4 | Did Reaction Recur on Re-administration (Study Drug) | For each study drug and related event | Yes | Study Drug |
| G.k.1 | Characterization of Drug Role (CM) | Site user opinion on the medication. Only inside a repeating item group with an item to form link to the concomitant medication form. | No | Item to Form Link |
| G.k.8 | Action(s) Taken with Drug (CM) | Site user opinion on the medication. Only inside a repeating item group with an item to form link to the concomitant medication form. | No | Item to Form Link |
| G.k.9.i.2.r.3 | Result of Assessment | Site user opinion on the medication. Only inside a repeating item group with an item to form link to the concomitant medication form. | No | Item to Form Link |
| G.k.9.i.4 | Did Reaction Recur on Re-administration (CM) | Site user opinion on the medication. Only inside a repeating item group with an item to form link to the concomitant medication form. | No | Item to Form Link |
Enablement & Configuration
This feature is automatically enabled and immediately available for configuration in the for studies using the E2BLink. Configuration for a live study might result in follow-up safety messages for existing Safety Cases.
Safety-EDC Connection: New Standard Safety Fields on the Study Drug Safety Form Type 25R2.3
Use Case
To better support device studies, we added new Standard Safety Fields for mapping to the Study Drug safety form type.
Description
In Studio > Form Configurations, the following fields are now available for mapping on the Study Drug form type:
- Gestation Exposure Number (SD_GESTATION)
- Gestation Exposure Unit (SD_GESTATION_UNIT)
- Device Name (SD_DEVICE_NAME)
- Device Evaluated (SD_DEVICE_EVALUATED)
- Device Usage Type (SD_DEVICE_USAGE_TYPE)
- Device Implanted Date (SD_IMPLANTED_DATE)
- Device Explanted Date (SD_EXPLANTED_DATE)
- Device Age (SD_DEVICE_AGE)
- Device Age Unit (SD_DEVICE_AGE_UNIT)
- Additional Information Coded (SD_ADDITIONAL_INFO_CODED) - If Each Entry In is set to Form, this field allows for the mapping of EDC Items in both repeating and non-repeating Item Groups.
If the form configuration has Each Entry In set to Repeating item group, all Items must be in the same EDC repeating Itme Group as the Start Date (SD_START_DATE) item.
If different answers are recorded on multiple EDC forms for the Study Drug or Device, EDC will transfer the last modified value. This is required to adhere with the E2B-compliant product information in Veeva Safety.
Enablement & Configuration
This feature is immediately available for configuration for studies using the Safety-EDC Connection.
Safety Integration: Suppress Study Drug When Classified Not Administered 25R2.2
Use Case
Adverse Events can be recorded before a study drug has been administered. Prior to this release, the study drug details were transferred with a Safety Case with a drug role of “Drug Not Administered” (E2B code=”4”) even if the study drug had not yet been dispensed . Now, a form configuration setting on the Study Drug safety type is available that will suppress the transfer of the study drug information if it has not yet been administered. This will reduce noise in the Safety Case processing in the safety system.
Description
In E2BLink, if the study drug information is embedded on the Safety Case Initiation Event (for example, the Adverse Event form) and when using “Each Entry In: Repeating item group in form”, the new setting will be available in the Inclusion Criteria tab.
While the setting will be set to “No” for all existing studies and Form Configurations, it will default to “Yes” for new studies and new Form Configurations.
Enablement & Configuration
For testing in Limited Releases, contact Support to enable this feature.
This feature is automatically available for configuration in the General Release for studies using either the Safety-EDC Connection or the E2BLink. Configuration for a live study might result in follow-up safety messages for existing Safety Cases.
EDC API
The following are new features for the EDC API. See our Developer Portal's release notes for more detailed feature information.
EDC API Features 25R2.3
This release includes the following features for EDC developers:
- Manage Protocol Deviations
- Start and Manage Vault-Level Jobs
- Support for Minimum Number of Repeating Forms
CDB API
The following are new features for the CDB API. See our Developer Portal's release notes for more detailed feature information.
CDB API Features 25R2.3
This release includes the following features for CDB developers:
- Study File Format Package Versioning
- Study File Format: Ensuring Consistent Form Submit Dates
EDC Migrator
Features in this section are new features for Veeva EDC Migrator.
Support for Migrating Fasting Status & Female Cycle 25R2.2
Use Case
The 25R2 EDC release introduced Local Lab support for Fasting Status and Female Cycle parameters in lab normal range management. This release extends that support to studies migrated from other EDC systems.
Description
This feature supports migrating lab source data with Fasting Status and Female Cycle parameters. For lab forms with Fasting Status and/or Female Cycle enabled in Studio, EDC Migrator will provide mapping and validation support for the new lab header (which was introduced in 25R2). This includes YAML Builder mapping support.
Enablement & Configuration
This feature is immediately available for studies being migrated.
YAML Builder Support for Item Units 25R2.2
Use Case
Previous enhancements supported mapping for “unit” item types but required you to manually create them. With this update, the YAML Builder automatically inserts these mappings.
Description
The YAML Builder inserts mappings into the applicable form YAML files for items that are units.
Enablement & Configuration
This feature is immediately available for studies being migrated.
Support for Form & Item Linking 25R2.3
Use Case
This feature greatly reduces the amount of manual intervention required to create links in migrated studies.
Description
The EDC Migrator can now create form-to-form and item-to-form links using a Links CSV file.
A single Links CSV file is used for both form-to-form and item-to-form linking. In the file, the columns required for the parent form links have “_REF” appended to the column header. Columns required to associate the other parent form have “_LINK” appended to the column header. Required columns must be populated, otherwise, an error occurs.
Enablement & Configuration
The study design must be configured with form links, and the external Links CSV file must be included with the clinical source data.
Learn More
Lab Data Application Enhancements 25R2.3
Use Case
We’ve introduced two lab enhancements to support migration use cases when a lab Collection Datetime was entered and then removed, appearing as empty in the source data, and when a migrating study uses lab normal range overrides.
Description
The EDC Migrator can now successfully send empty lab Collection Datetime values. In these cases, the resulting lab collection form displays header information, but the lab results are uneditable until a supported time is provided. This is consistent with manually entered forms where the Collection Datetime is not provided or is removed. Also, when a migrating study uses lab normal range overrides, the reason for the override is now included in the audit trail.
Enablement & Configuration
These updates are immediately available in migrating studies with local lab data.
Learn More
Support for Lab Data Migration from InForm™ 25R2.3
Use Case
The YAML Builder now supports mapping lab data when migrating studies from Inform to Veeva EDC.
Description
Migration users can now use the YAML Builder to create mapping files for studies that originated in Inform and contain lab data.
Enablement & Configuration
This feature is immediately available.