What's New in 24R3
Pre-Release Date: November 11, 2024 | CDB Pre-Release Date: November 18, 2024 | Release Date: November 22 & December 6, 2024We are pleased to bring you Veeva Clinical Data in 24R3. Read about the new features below. You can find information on enabling new features in the 24R3 Feature Enablement Details. Information on developer features (REST API) is in the Developer Portal.
Clinical Data
Features in this section are changes that apply to all application areas of Veeva EDC and CDB.
Vault EDC Imaging
Use Case
This feature supports the collection of DICOM exams in a study, integrating the process of uploading and viewing Imaging Exams and associated data with Vault CDMS, and adding a new Imaging tab that allows sponsors oversight of uploaded exams.
Description
This release introduces Vault EDC Imaging, which allows Imaging data gathered in the course of your Study to be shared across Vault EDC modules including the Review tab and the new Imaging tab. Imaging information is retained in the same system as the rest of study data, which offers benefits such as reducing the need for data reconciliation, making sponsor oversight more efficient, and optimizing workflows.
Vault EDC Imaging supports the following capabilities:
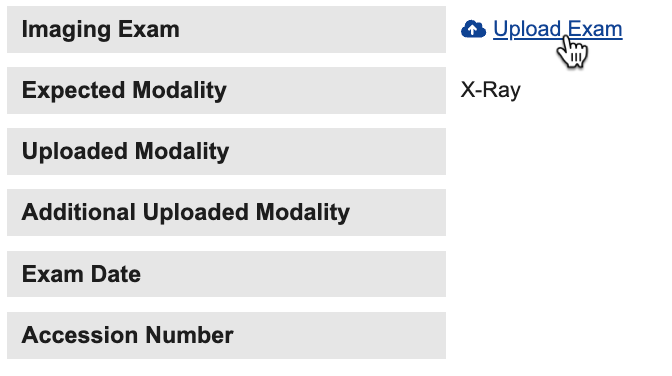
Upload & Validation of DICOM Exams
Site users can upload through forms in Data Entry. Before upload, the system automatically validates the exam data to ensure its integrity. The uploader is required to confirm that the exam does not contain sensitive patient information. The system automatically de-identifies file names and DICOM® tags to minimize the risk of retention of protected health information (PHI) or personally identifiable information (PII). System-managed Imaging Items automatically collect key metadata when the DICOM® imaging exam files are uploaded.
During the upload process, the user can continue working freely within Data Entry.
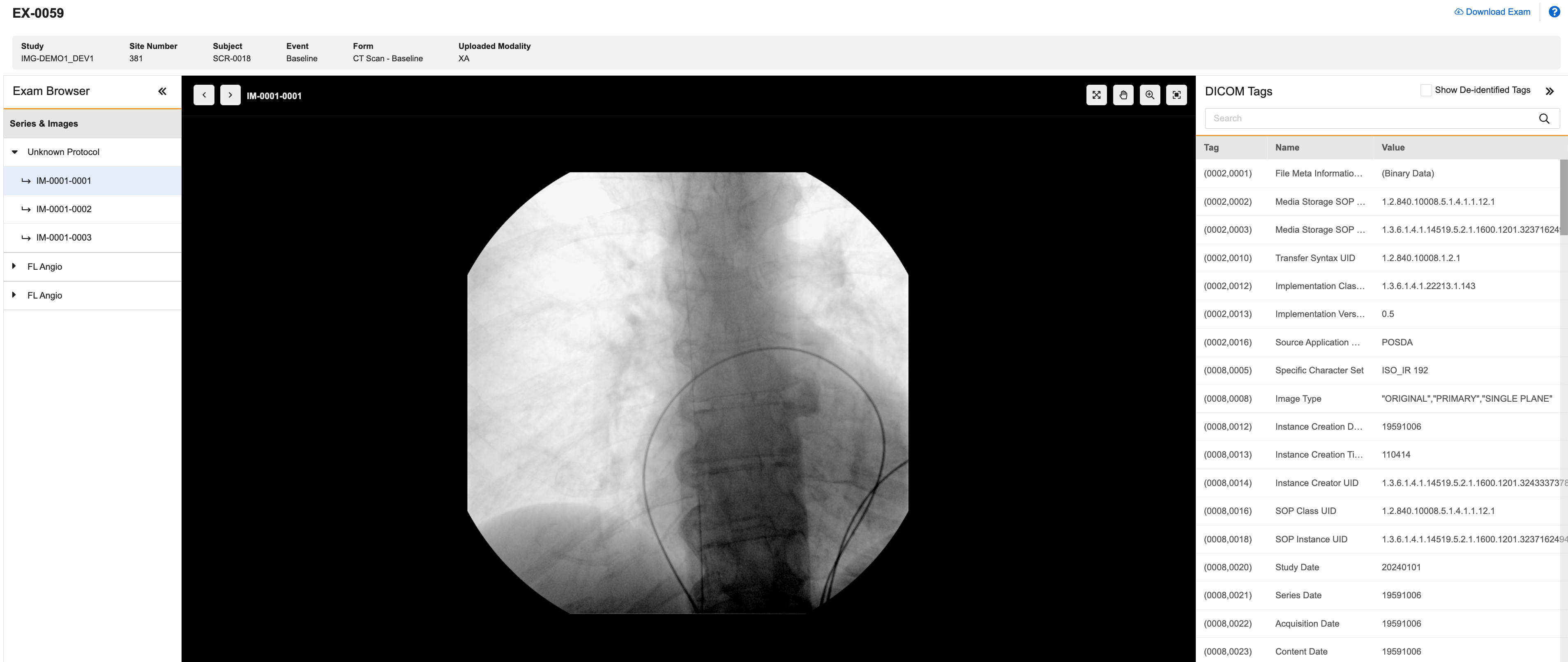
Vault EDC Imaging Integration
A new Imaging tab allows users with the CDMS Imaging Specialist role to view and download imaging exams. After an exam is uploaded, it can be viewed from several locations in Vault EDC, including the Data Entry tab, the Review tab, and the Imaging tab. Users with access to the Review tab can perform SDV/DMR tasks and create queries on Imaging Exam items.
Imaging Exam Configuration
Study designers can configure forms within the casebook to include Imaging Exams through Studio. The new Imaging Items and Imaging Item Group study objects allow Study Designers to select an Expected Modality when creating the Imaging exam. Study designers can add one Imaging Exam per Form.
Imaging Roles & Permissions
When Imaging is enabled at the vault level, a new role, CDMS Imaging Specialist, is added to the vault. CDMS Imaging Specialists and users with permissions have access to the Imaging tab, where they can view and download Imaging exams that have been uploaded in Data Entry. Other new Imaging permissions have been added, including permissions to upload Imaging Exams and review Imaging Exams. Permissions for the following existing standard roles have been changed to include one or more of the new Imaging permissions:
- CDMS Super User
- CDMS User Administrator
- CDMS API Read Write
- CDMS Librarian
- CDMS Study Designer
- CDMS Clinical Research Coordinator
- CDMS Principal Investigator
- CDMS Sub Investigator
This feature is available only to early adopters. Note that the Study Design Specification (SDS) reflects new fields related to Vault EDC Imaging configuration that will display for all customers.
Enablement & Configuration
This feature is available for early adopters. Contact Veeva Services for more information.
Learn More
Accessible Color Palette
Use Case
These updates meet WCAG color contrast standards to support persons with color vision deficiencies including color blindness.
Description
Many updates have been made to the CDMS color palette in order to comply with accessible color contrast guidelines. This is the first step in a broader effort to improve the accessibility of Vault CDMS.
- Blue primary buttons are a more vibrant blue.
- EDC task and status icons use more saturated colors.
- Hyperlinks are a bolder blue and appear underlined when hovered.
- The default text color was darkened.
Enablement & Configuration
This change applies automatically.
Data Entry
Features in this section are changes to the Data Entry tab, a working area for investigators and clinical research coordinators to enter study execution data.
Undo SDV/DMR on Event, Form, and Item Group Reset
Use Case
This enhancement keeps reset behavior consistent for items with and without values, where the SDV/DMR will be set to false when the form is reset.
Description
After this release, when the Reset action is performed, the SDV and DMR that was applied will be automatically set to false in cases where items have blank values. Previously, if SDV and DMR had been set on items that were left blank, the SDV and DMR had remained set as true when the site performed a reset action. As the sites re- entered data on the events, forms and items, then the SDV and DMR were automatically updated to false.
- Event Reset: the SDV/DMR is cleared from the Event Date and Visit Method
- Form Reset: the SDV/DMR is cleared from all items on that form
- Item Group Reset: the SDV/DMR is cleared from the items in that item group
Event and form data that is populated by APIs as external owned sources will remain, and therefore any SDV and DMR will also remain, unless the user clicks “Reset External Data Values”.
Enablement & Configuration
This feature is automatically available.
EDC Numeric Value Conversions
Use Case
The entry of full-width numeric characters into EDC causes ingestion errors for downstream systems that can’t ingest the embedded style of these characters. Downstream systems can expand numbers in scientific notation to the full value upon ingestion which doesn’t conform to the configured length and precision for items. The transformations and validation introduced by this update prevent errors downstream caused by unsupported data.
Description
This release includes two updates to numeric value formatting and conversions to support EDC data ingestion to CDB and Clinical Reporting:
- When full-width numeric characters are entered into EDC, they will be converted to half-width numeric characters when the data is saved. This conversion only occurs for number fields.
- Scientific notation is not a supported format for EDC number fields. We have added a validation in data entry to not allow scientific notation.
Enablement & Configuration
These updates are automatically available for studies using Data Model 2.
Learn More
Data Review
Features in this section are changes to the Review tab, a working area for clinical research associates and data managers, or to review functionality within the Data Entry tab.
Show Blank Repeating Forms in the Review Tab
Use Case
This feature provides clarity to CRAs and Data Managers by allowing them to see that a repeating form is available for the site to fill out.
Description
Prior to this release, Vault only showed repeating forms in the Review UI when data was present. With this release, the Review UI display will match the Data Entry display. An empty form icon will display, along with an indicator that no form instances exist. When selected, the form name will show in the right panel with an indication that there are no records to display.
Enablement & Configuration
This change applies automatically.
Direct Query Navigation Updates
Use Case
CRAs and DMs can more easily navigate to queries from reports, allowing them to immediately take action on queries in Review.
Description
Users will now be directed to the query location in Review when selecting the Query Name or Query Message (VV-#####) from within a report.
Enablement & Configuration
This feature is automatically available.
First Event SDV/DMR Completion Date
Use Case
This update provides CRAs and other Review users the ability to see the date when SDV was first completed at an event.
Description
In this release, we’ve added additional new columns to the Event Progress Listing for First Event Review Date and implemented the following changes:
- First Event SDV Complete Date and First Event DMR Complete Date columns display the initial date when SDV/DMR is complete for all forms, event date, and visit method (where required). The value is a roll-up of Review State > First Review Date
- After being set, the First Event Review Date will not be updated if a review value changes, however, it will be cleared if the Event is reset
- The roll-up will be re-evaluated if review states are added, removed, or updated
- For studies where First Review Date is enabled, the First Event SDV/DMR Completed Date value will be backfilled for all events since the 23R3 release when First Review Date began being captured
Enablement & Configuration
These updates are available for studies on Review Rollup V2 with First Review Date enabled.
More Granular Time Calculation for Query Caused Data Change
Use Case
Evaluating the timing down to the level of seconds ensures a more granular and accurate inclusion of these cases within the listing.
Description
Previously, in rare cases, items were not included in the Query Caused Data Change field of the Query Detail Listing when data was changed within the same minute that the query was opened. The calculation now considers seconds when determining if the query caused the data to change, and more accurately reflects these cases within the listing.
Enablement & Configuration
This feature is automatically available.
Synchronous Creation of Review States
Use Case
This update improves the user experience by preventing partial failures of the Reevaluate Plan Assignment job.
Description
This feature streamlines the creation of backend SDV and DMR records, creating them when an Item or Event is created. This change addresses past issues in which plan assignment was incomplete due to partial failure of the Reevaluate Plan Assignment job. With this release, if any part of the job fails, the entire job will fail.
Enablement & Configuration
Contact Veeva Support to enable this feature in the 24R2.3 limited release.
Clinical Coding
The following are new features for Veeva Coder, the clinical coding area for Veeva Coder.
Coder - Allow Change of Dictionary Release & "Downversioning"
Use Case
Customers can now independently resolve issues related to selection of the wrong dictionary release or unintended upversioning.
Description
Coder now supports the ability to update the Dictionary Release field in Coder Tools > Study Settings even after code requests exist for this coding form configuration, as long as no coding has been completed. Additionally, Coding Forms can now be downversioned as well as upversioned. Downversioning can only be performed on individual forms from Coder Tools > Study Settings, and cannot be performed in bulk or for Synonym Lists. To support this change, display text in several areas (labels, email notifications, dialogs, etc.) has been updated to reference “Dictionary Versioning” when applicable for both Upversioning and Downversioning actions.
Enablement & Configuration
These updates are automatically available.
Learn More
Coder - Allow Approve Mode from List View
Use Case
This feature provides code approvers with the option to use List View during the approval process, which provides additional context including Coded By, Notes, and Queries.
Description
Coder now supports the use of Approve Mode while in List View. When a Coder User switches to Approve Mode, the system will no longer auto filter to codes that are in the Pending Approval status. When a user approves a code request with the Apply to Synonym List option checked, any new synonym list detail records that are created will be in the Active status, without need for further approval. In Approve Mode, the Dictionary and Suggestions subtabs in the Coding panel have been added in a read only format.
Enablement & Configuration
These updates are automatically available.
Learn More
Coder - Set Coding from External Suggestions
Use Case
External coding suggestions received via API can now be used as a first-line coding solution.
Description
The external coding suggestions functionality is enhanced to allow direct coding of a verbatim from a posted suggestion. Using the API Set Coding Suggestions endpoint, one external suggestion source can be flagged to set coding. If the verbatim is neither excluded by the Do Not Autocode List nor already auto-coded from the Dictionary or Synonym List, the flagged suggestion will be used for auto-coding. To enable direct coding from an external suggestion for a study, the study must have Enable Approval Workflow set to Yes in Coder Tools > Study Settings. After the verbatim is autocoded with the applied external suggestion, the verbatim will have the status of Pending Approval, requiring review by a user. While this introduces significant efficiencies, the approval workflow is required to review AI-supported external coding to enforce regulatory compliance. Externally autocoded terms can be added to the synonym list, for further improvement to efficiency.
Enablement & Configuration
These updates are not automatically available (behind feature flag), and will only be enabled upon request from the client.
Learn More
Coder - Dictionary Versioning Impact Report Enhancements
Use Case
These updates improve usability and readability.
Description
This release introduces several enhancements to the Impact report available when dictionary versions and Synonym Lists are updated. Changes include:
- A Study column has been added to the Form Impact report.
- Highlighting and text formatting have been added to identify each cell that changed in the source (red, italicized) and target (green, bolded) release for a verbatim that was remapped.
- Translations have been added for all Impact Report Column Header labels and the Summary tab.
Enablement & Configuration
These updates are automatically available.
Study Design & Configuration
Features in this area apply to Studio, the study design and configuration area for Veeva EDC.
Compress and Flatten PDFs
Use Case
This feature helps customers who are merging or copying pages from multiple PDFs by minimizing the file size for both the zip and the extracted files, which helps with overall performance.
Description
There is now a reduction in file size when generating the Blank and Annotated PDFs from Studio. PDFs have been optimized in order to reduce file sizes by approximately 20-30%. The reduction in the PDF file size will vary per study.
Enablement & Configuration
This feature is automatically available.
Extract Enhancements: Queries in User Language
Use Case
This feature provides the user with translated query text in data extracts and reports.
Description
We’ve added columns that show the translated value of query text to the following extracts to support the Queries in User Language feature released in 24R2:
- Query Detail Listing Report v2 Template
- Query Detail Listing
- SDE SYS_Q and SYS_QT data sets
Enablement & Configuration
This feature is automatically available.
Learn More
Item Default Length for Codelists
Use Case
The default item length has been made smaller to reduce the character lengths during the design of the study while still providing flexibility for designers to adjust the item length as needed.
Description
When creating an Item or changing an Item Type to a Codelist data type in Studio, the length of the Item will default to 100. If required, the item length can be further revised to reduce or expand the item length per the values from the codelist.
Enablement & Configuration
This feature is automatically available.
Required Item Query Options
Use Case
This feature provides Study Designers the flexibility to select how the item’s required query will operate when an item or form is marked ILB.
Description
With this feature, we’ve added additional options to further specify when queries should fire when something is marked as Intentionally Left Blank (ILB), if configured. When configuring automatic queries in relation to the ILB functionality, Study Designers can specify when Vault should fire a query: when an Item is marked as ILB and/or when a Form is marked as ILB. In Studio, users can select from these options in the Edit Checks section of an item’s properties panel.
A new column for the Open Query field has also been added to the Studio Study Design Specifications (SDS) Excel™ and PDF.
Enablement & Configuration
This feature is automatically available. Existing studies where an item has the “Yes, fire query when ILB” option selected will operate with the same behavior, and both Open Query options will be selected for that item.
Studio Medical Coding Improvements
Use Case
Previously, medical coding configuration that was set to inactive or deleted caused errors when performing study copy actions or generating a difference report. This update helps to prevent errors during new study setup.
Description
When utilizing the study copying functionalities or running difference reports in Studio, Vault will ignore Medical Coding definition records that have been deleted or inactivated.
Enablement & Configuration
This feature is automatically available.
Blank CRF Update for Repeating Item Groups
Use Case
Previously, when generating the Blank CRF, any repeating item group that had no defaults configured showed five copies of the item group. Showing one instance of the item group helps reduce confusion around how repeating item groups with no defaults display within the Blank CRF, providing a more clear and concise rendition.
Description
When generating the Blank CRF from Studio, only one copy of the repeating Item Group will be created in cases where there is no default configured for the Item Group.
Enablement & Configuration
This feature is automatically enabled for new studies only.
Study Administration
Features in this section apply to System Tools or EDC Tools, a study-level administration area for Veeva EDC.
Data Extract Job Enhancements
Use Case
This error provides actionable information to the user as to why a scheduled job might fail, and how to address the issue.
Description
We’ve added an error message to the Core Listings job log when a Form is deleted or changed to Restricted for scheduled jobs.
New columns for the raw date and datetime (the date or datetime as entered by the site, without any timezone transformation) have also been added to both Core Listings and the 24R3 version of the Study Data Extract. The column name is the name of the item with ‘_RAW’ appended.
Enablement & Configuration
This feature is automatically available.
Deployment Date Added to Vault and Study Deployment History File Name
Use Case
This change makes it easier for users to identify which deployment downloaded Files and Logs are from.
Description
The timestamp of the deployment date is included in the file name when the File and Log are downloaded from the Vault Deployment or Study Deployment History pages.
Enablement & Configuration
This feature is automatically available.
EDC Event Progress Listing Enhancements
Use Case
These enhancements improve the usability of the Event Progress Listing job.
Description
- Added new columns to track Event-level SDV/DMR Required, Complete, Complete Date, and % Complete. These values will be blank for log events and will only populate for studies using Review Rollup V2
- Relabeled existing column for Event SDV/DMR Completion Date to Forms SDV/DMR Completion Date, as these columns only take forms into account.
- Added a new column for Marked for Removal
Enablement & Configuration
This feature is automatically available.
Learn More
EDC Form Progress Listing Enhancements
Use Case
These enhancements improve the usability of the Form Progress Listing job.
Description
The Form Progress Listing now includes a new Marked for Removal column.
Enablement & Configuration
This feature is automatically available.
Learn More
EDC Query Detail Listing Enhancements
Use Case
These enhancements improve the usability of the Query Detail Listing job.
Description
This feature includes the following updates to the Query Detail Listing:
- The Item Value Now column will now pull from the Item Data Change record to ensure optimal formatting for data entered after 23R3. Values entered prior to 23R3 will continue to pull from Item History
- Date formats for Item Value Now are now formatted as dd-MMM-yyyy to be consistent with Item Value Before Query
Enablement & Configuration
This feature is automatically available.
Learn More
EDC Subject Progress Listing Enhancements
Use Case
These enhancements improve the usability of the Subject Progress Listing job.
Description
The following changes have been made to the Subject Progress Listing:
- New columns have been added for Subject SDV/DMR Required & Completion Date. These values will only populate for studies using Review Rollup V2 & Data Model V2
- The SDV/DMR Complete columns have been relabeled to Subject SDV/DMR Complete. Additionally, the logic for these columns has been updated to use the Casebook Operational Summary instead of calculating from other columns in the listing for studies using Review Rollup V2 and Data Model V2
- We have updated the logic for displaying the Frozen, Locked, and Signed columns for studies using Data Model V2. We now pull from the casebook summary record
Enablement & Configuration
This feature is automatically available.
Learn More
Job Selections Retained After Validation Error
Use Case
This change makes it easier to correct selections when saving scheduled jobs.
Description
If a validation error occurs when a scheduled job is initially saved, the selections are no longer cleared out prior to saving, allowing the user to adjust the settings.
Enablement & Configuration
This feature is automatically available.
Copy to PPT Job Does Not Include Signature History
Use Case
This change excludes records from the copy to PPT operation that could cause the job to fail.
Description
When data is copied to Post Production Test (PPT) environments, Signature History records will no longer be included as part of the copy operation.
Enablement & Configuration
This feature is automatically available.
Rules Enhancements: Show Sequence Numbers in EDC Tools
Use Case
Sequence numbers were not previously displayed in EDC Tools. Sequence numbers provide users clarity on how a rule operates when viewing rules and managing rule jobs in EDC Tools.
Description
Sequence numbers in rule actions that pertain to specific instances of repeating Event Groups, Forms, and Item Groups are now displayed in the dialog when viewing rule details in EDC Tools.
Enablement & Configuration
These feature is automatically available.
Enhanced Options when Running User Reports
Use Case
These enhancements provide additional selections and usability improvements with the User Reports.
Description
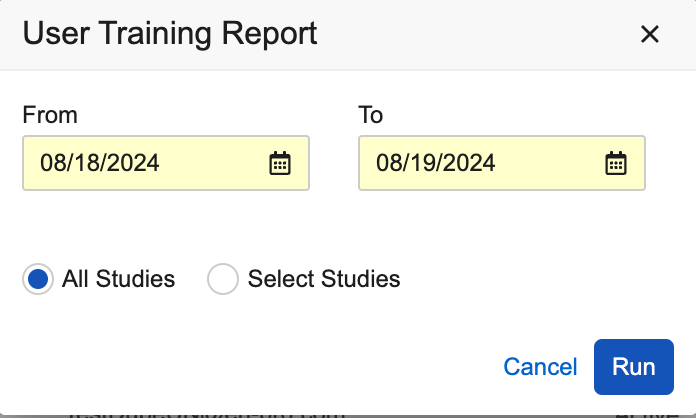
The following enhancements have been added for User Reports:
- A date picker has been added as an option when running the User Training Report, allowing users to select a range for the ‘Date Assigned to Study’ values in the report.
- When “Select Studies” is chosen while running the User Training, User Access, and User Activity reports, the selected studies are displayed in alphabetical order.
Enablement & Configuration
These updates are automatically available.
Learn More
Vault Configuration Report File Name Update
Use Case
Including the Vault Name in the file name provides clarity on which Vault this report was generated.
Description
The format of the file name has been updated when running the Vault Configuration Report. It now includes the Vault name. The new format is as follows:
Vault_Configuration_Report_$VaultName_$Date-$Time-$TzAbbr.xlsx
Enablement & Configuration
These updates are automatically available.
Vault Level FTP Connections Configuration
Use Case
This feature allows external connections for exports to be defined in one location, with one username and password to update if needed, and can be accessed by all studies on the vault.
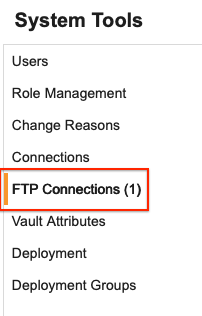
Description
We’ve added a new page for FTP Connections in System Tools that allows Vault Administrators to create connections to FTP servers that can be used by all studies. In EDC Tools, Vault-level connections will appear as a new group so that the connection can be utilized by a study job. When defining an export from EDC Tools, a new section in the dropdown will show available study and vault connections.
Enablement & Configuration
This feature is automatically available and can be configured in System Tools.
Learn More
Verbose Option for Audit Trail Export
Use Case
This feature provides continuity with older audit trail exports.
Description
A checkbox to “Include Definition, Relationship, and Facade Audit Changes” is now available under Export Options when scheduling one of the Audit Trail Export jobs. This option allows users to exclude (unchecked) or include (checked) extraneous change records that were suppressed in the 24R1 enhancement. This setting can be used with the following exports:
- Audit Trail Export By Subject
- Audit Trail Export By Site
- Audit Trail Export by Study
Enablement & Configuration
Contact Veeva Support to enable this feature.
Learn More
Labs
Features in this section are new features for the Labs module of Veeva EDC.
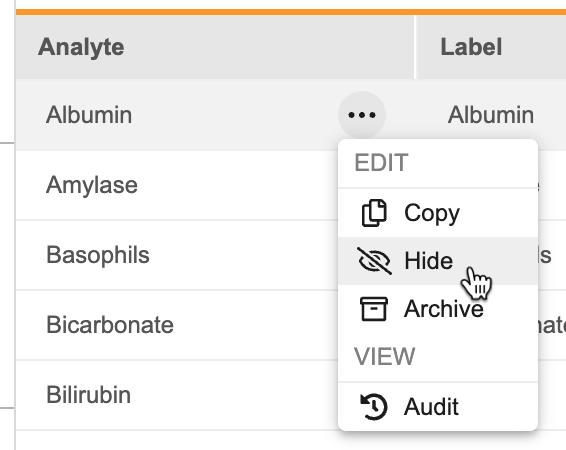
Labs: Hide Analyte Functionality
Use Case
This update provides sponsors with additional flexibility to prevent future use of an analyte. Previously, analytes could only be archived, which is not supported once an analyte is in use.
Description
Users can now hide analytes in the Analyte Library in DEV to prevent an analyte from being selected in future Lab Panel configurations. Existing uses of the hidden analyte are not impacted and can continue collecting data. As part of this update, the Analyte Library export has been updated to include the Analyte Status. This column will be ignored if used in an analyte import.
Enablement & Configuration
This update requires enablement by Veeva Support for limited releases, but will be immediately available in 24R3 general release. The Analyte Library export update is immediately available with the release of 24R2.2.
Learn More
Role Management & Security
Features in this section are enhancements to the System Tools > Role Management and System Tools > Users areas, as well as changes to standard Study Roles, security, and access control in Veeva Clinical Data.
UI Updates to Role Management
Use Case
These enhancements improve usability to provide a better user experience.
Description
The following improvements have been made to the Role Management area:
- The CDMS Auditor Read Only role now has the View Query permission checked which was inherently part of the View Casebook permission and needed to view query data in the Query Detail Listing report.
- By default, the Role Management page in System Tools has all groups expanded.
- When expanding or collapsing role groups, the state of the groups is remembered in the UI.
- Clicking a cell within the Role Management grid will highlight that row, column, or role/permission intersection. You can click the cell again to remove the highlight.
- The “Number of Users” label has been changed to “Number of User Assignments” to better describe the information contained therein.
- The “User Assignments” dialog now contains all applicable user assignments, regardless of whether the user’s study access is enabled or not.
Enablement & Configuration
These updates are automatically available.
Learn More
New Standard Study Roles for CDB
Use Case
New standard roles allow customers to reduce the number of custom roles they need to create, and reduce the testing burden with each release. Standard roles are validated, and new permissions may be added as new functionality is incorporated into the application.
Description
This release provides a new standard role to support CDB processes: CDMS CDB Read Only.
CDB Read Only can access both the Workbench and Clinical Reporting applications, allowing a user to view listings, and import and export information, but without the ability to create or mark listings as reviewed, to create queries, or to create export definitions.
Enablement & Configuration
These new roles are available to be assigned to users after the release.
Connections & Integrations
Features in this section are new connections or integrations with Veeva Clinical Data or enhancements to existing ones.
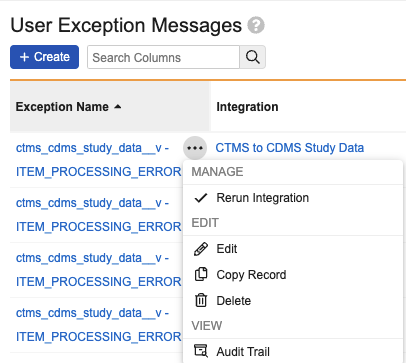
Clinical Operations-EDC Connection: Manual Data Refresh
Use Case
Users will be able to correct issues in EDC that are causing errors with the Connection, such as a mapping issue for Country or Site. They can then immediately resolve discrepancies, preventing potential downstream issues.
Description
To support the management of the Clinical Operations-EDC Connection, EDC Vault Owners can now manually trigger an integration refresh. A new Rerun Integration menu option will be available in Admin > Connections > User Exception Messages. Triggering the rerun will re-sync EDC with CTMS data on demand, as if a change had been made in CTMS that would have automatically triggered the refresh. This feature includes a corresponding functionality in CTMS at the User Exception Message level, allowing the reprocessing of a Study Refresh Request.
Enablement & Configuration
This feature is automatically enabled for studies using the Clinical Operations-EDC Connection.
Learn More
Clinical Operations-EDC Connection: Automatically Match Countries by Country Code
Use Case
The auto-mapping of countries using the Vault-wide country code significantly reduces the effort involved in connection setup across environments and lowers the risk of potential discrepancies.
Description
The country mapping of the Clinical Operations-EDC Connection now leverages the standardized country codes across Vaults. Manual country mappings between CTMS and EDC will remain intact for existing connections and can still be used to override auto-mapping. The new functionality is recommended for establishing new connections and adding countries to existing setups.
Enablement & Configuration
This feature is automatically enabled for studies using the Clinical Operations-EDC Connection.
Learn More
Safety-EDC Connection: Severity
Use Case
Expanding the set of standard fields for mapping from Vault EDC to Vault Safety further increases the ease of use of the Safety-EDC Connection.
Description
For the Safety-EDC Connection, Severity was added as a new standard field for mapping on the Adverse Event Form Type.
Enablement & Configuration
This feature is automatically enabled for studies using the Safety-EDC Connection. Note: Configuration for a live study might result in Follow-Up Safety Messages for existing Safety Cases.
Safety-EDC Connection: Multi-Vault Support
Use Case
A single Vault Safety might be used to manage data from several different EDC systems. To increasingly use the efficiencies of Vault Safety, the Safety-EDC Connection can now be established from one Vault Safety to one or more Vault EDC instances. The information is marked with the EDC source and can be managed accordingly in Vault Safety.
Description
The Safety-EDC Connection can now be established between one Vault Safety and multiple Vault EDC while they are hosted on the same Domain. The interchange of information between Vault Safety and each Vault EDC will be restricted to only those records that belong to the respective Vault EDC. Safety cases are identified in Vault Safety and attributed to the appropriate Source Vault EDC for Safety Case management.
Enablement & Configuration
Contact Veeva Support to enable this feature.
Safety Integrations: Study Drug information by Subject Group
Use Case
For studies using Arms, Cohorts and Substudies, usually multiple drug exposure forms are being configured to cover all study drugs. While subjects are never exposed to a subset of those study drugs, the respective information will still be transferred as “Drug Not Administered”. With the option to select Study Group-specific information for transfer, only the relevant drug exposure information for the given subject will be added to the Safety Case
Description
The inclusion of study drug information into a Safety Case can now be based on Subject Groups. When configuring the Study Drug Form Type and mapping to the respective study drug exposure forms in EDC, the ‘Only Include When Subject Group is’ setting can be used as a new filter for study drug data inclusion into the Safety Case.
Enablement & Configuration
This feature is automatically enabled for studies using the Safety-EDC Connection or E2B Link. Note: Configuration for a live study might result in Follow-Up Safety Messages for existing Safety Cases.
Safety Integrations: Details of the Reporter as Primary Source of Information
Use Case
Entering site information for each safety case when reporting an adverse event is burdensome for the site. Maintaining this information in EDC and submitting it automatically with each case will reduce redundant work and improve the data quality. Additionally, keeping the information of the first reporter in place will ease compliance with regulatory requirements.
Description
In the Safety Settings in Studio, “Principal Investigator Only” (previously “Principal Investigator”) or “Full Site Information” can now be chosen as Reporter. Accordingly, either the Principal Investigator Name and Country only or now additionally Organization (C.2.r.2.1), Address Lines (C.2.r.2.3), City (C.2.r.2.4), State (C.2.r.2.5), and Postal Code (C.2.r.2.6) of the initial reporter are transferred with each Safety message. For all new Safety Cases after 24R3, the Reporter information will be marked as unchangeable for all Safety Messages after the first send for both the Safety-EDC Connection as well as the E2B Link. If changes to erroneous Reporter details are required after the first send, please contact Veeva Support. For the Safety-EDC Connection the transfer will prioritize any Reporter information that is mapped for the Adverse Event Form Type to Adverse Event form items. When using the Adverse Event Form Type mapping, the Connection additionally allows the transfer of Email and Phone number.
Enablement & Configuration
This feature is automatically enabled for studies using the Safety-EDC Connection or E2B Link. Note: Configuration for a live study might result in Follow-Up Safety Messages for existing Safety Cases.
Safety Integrations: Study Type of Reaction & Medical Confirmation by Healthcare Professionals
Use Case
Expanding the general Safety Settings and the standard data transfer options for studies using the EDC Safety integration complements the information that is sent to the Safety system and thereby reduces the burden for Safety users to manually set these values for each incoming Safety Case.
Description
The Safety Settings in Studio for both Safety-EDC Connection and E2B Link studies have been expanded to include the following settings and functionality. The Study Type of the Reaction (C.5.4) can now be specified as “Clinical trials”, “Individual patient use” or “Other studies”. “Clinical trials” will be set automatically as the new default without causing Follow-Up sends for any Safety Case of live studies. The Auto Set Medically Confirmed will determine the inclusion of the Medical Confirmation by Healthcare Professionals (E.i.8). “No” will be set automatically as the new default, omitting this addition to the Safety Message and not causing Follow-Up sends for any Safety Case of live studies.
Enablement & Configuration
This feature is automatically enabled for studies using the Safety-EDC Connection or E2B Link. Note: Changing the default Study Type (C.5.4) or the Auto Set Medically Confirmed setting for a live study will result in Follow-Up Safety Messages for all existing Safety Cases.
E2B Link: Standardized EDC Data Transfer via Reporter or Sender Comment
Use Case
Not all safety-relevant EDC data can be transferred to a Safety system when using the E2B format. Adding this EDC information in a standardized way to the Report or Sender Comment field will support the transfer of non-E2B data values and reduce the need for the Safety team to access EDC data by other means than the E2B Link.
Description
Sending data from EDC to a 3rd party Safety system is based on the transfer of E2B XML files. The E2B format has limitations in regards to the data fields and formats that can be sent from EDC to a Safety system (e.g. Cohort or Toxicity Grade). This information might, however, be critical for the Safety team to assess a Safety case. To overcome the E2B restrictions, we now offer the mapping of EDC items to the Reporter Comments (H.2) or Sender Comments (H.4) field in the E2B XML. The values of the selected items for mapping will be added and listed as Notes in a standardized way to either Comment field, and appended to any actual Reporter or Sender Comments text. The setup is identical and similarly straightforward as for the general Safety Form Types. The new configuration options will be reflected in the SDS.
Enablement & Configuration
This feature is automatically enabled for studies using the E2B Link. Note: Configuration for a live study might result in Follow-Up Safety Messages for existing Safety Cases.
E2B Link: Study Drug Indication & Pharmaceutical Dose Form
Use Case
Expanding the set of configurable and mappable fields on Safety Form Types increases the flexibility to transfer EDC data using the E2B Link.
Description
On the Study Drug Form Type, a Study Drug Indication can now be either specified as a static value or mapped to an item value. The static value will be applied to all Safety Cases in the study. The mapped item must be unique in the subject’s casebook and will be transferred for all Safety Cases of the subject. Pharmaceutical Dose Form (G.k.4.r.9.1) is now available for mapping on the Concomitant Medications Safety Form Type.
Enablement & Configuration
This feature is automatically enabled for studies using the E2B Link. Note: Configuration for a live study might result in Follow-Up Safety Messages for existing Safety Cases.
E2B Link: NullFlavor for Name, Source and Method of Study Drug & Additional Document Availability
Use Case
Expanding the general Safety Settings and the standard data transfer options for E2B Link studies complements the information that is sent to the Safety system and thereby reduces the burden for Safety users to manually set these values for each incoming Safety Case.
Description
For E2B Link studies, the Safety Settings in Studio have been additionally expanded to include the following settings and functionality:
-
The Name or Initials nullFlavor (D.1) can now be specified as MSK, ASKU, NASK or UNK. Not asked (NASK) will be set automatically as the new default for new studies.
-
The Source of Study Drug Assessment (G.k.9.i.2.r.1) and Method of Study Drug Assessment (G.k.9.i.2.r.2) can now be added as a static value. Any set value will appear in the E2B XML together with the Result of the Assessment (G.k.9.i.2.r.3), which can be mapped on the Study Drug or Concomitant Medications Form Types.
-
Additionally, the indication for Additional Document Availability (C.1.6.1) will now be transferred as “false” with every new Safety message without causing Follow-Up sends for any Safety Case of live studies.
Enablement & Configuration
This feature is automatically enabled for studies using the E2B Link. Note: When modifying any Safety Settings the default Name or Initials nullFlavor (D.1) will be a required field. Saving the new nullFlavour default or adding Source/Method of Study Drug Assessment for a live study will result in Follow-Up Safety Messages for all existing Safety Cases.
E2B Link: Safety Case ID from ACK file in EDC UI
Use Case
While the Safety-EDC Connection was built to back-propagate the Safety Case ID from Vault Safety to Vault EDC, external Safety systems do not natively allow for this. By using the information from the ACK file, we now also enable the E2B Link to display the Safety Case ID to Site users, and ease communication with Sponsor personnel on safety information.
Description
With the release of the Safety Case Banner, site users receive feedback on their submitted safety cases directly on the adverse event form, including the CDMS case ID and the Last Send datetime. The information will now be supplemented also for the E2B Link with the Safety Case ID as returned with B.r.7 from the ACK file. If available, the exact text from B.r.7 will be extracted and presented as Safety Case ID in the Safety Case Banner to EDC users with the “View Safety Case” permission. Note, only standard ACK files from the Argus safety system are considered and no other text from the ACK file can be inserted as Safety Case ID.
Enablement & Configuration
This feature is automatically enabled for studies using the E2B Link.
EDC API
The following are new features for the EDC API. See our Developer Portal's release notes for more detailed feature information.
EDC API Features
This release includes the following features for EDC developers:
- Retrieve Event Definitions
- Retrieve Event Group Definitions
- Set Coding Suggestions
Clinical DataBase (CDB) & EDC Clinical Reporting
The following are new features for the Veeva CDB application, EDC Clinical Reporting (the Veeva Clinical Data solution for data cleaning and reporting), or both.
Availability: Clinical DataBase (CDB) is only available to CDB license holders. Contact your Veeva Services representative for details.
Listings Enhancements for Builder and Grid
Use Case
These updates provide additional information to include in a listing, or to help when defining a union with identical item names.
Description
Listings in CDB Workbench and Clinical Reporting have been updated with the following enhancements:
- In the Listing Builder, codelist items now have the option to include the decoded value.
- When multiple items with the same name are included in a listing, the item group name has been added to the display in the Listing Builder Columns section to provide clarification of where each item is used.
- New options have been added to filter using Not In functions for text and numeric data types. These options are available in both the Listing Builder and the listing grid display.
Enablement & Configuration
These enhancements are automatically enabled.
Learn More
- Creating Listings, Checks & Views with the Listing Builder
- Creating Custom Listings
- Clinical Query Language (CQL) Reference
Display Indicators for Disabled Review Listings
Use Case
Improved visibility to review listings that are disabled or at risk of becoming disabled enables users to more quickly identify these long running listings and take action to improve the performance of the listing.
Description
For long-running Review Listings that have been disabled, we added additional indicators:
- In a Review Listing, we added iconography to show if the listing is disabled or at risk of becoming disabled.
- In the Review tab, we added review warning icons.
- On the dashboard, we moved the warning to the left of the Review Listing title.
- On the dashboard, we added quick filters for review listings that are disabled or at risk of becoming disabled.
Enablement & Configuration
This feature is enabled automatically.
Learn More
New Columns for Sys_Events, Sys_Forms & Sys_ILB Call Functions
Description
With this release, we’ve added new columns to the following CALL functions:
- Sys_Events:
- Study.Label
- Site.CountryName
- EventGroup.Label
- EventGroup.ExternalID
- Event.Label
- Event.ExternalID
- Event.VisitMethod
- Event.PlannedDate
- Event.EventDateModifiedDate
- Casebook.Version
- Event.ChangeReason
- Event.ExpectedForms
- Event.Frozen
- Event.FrozenDate
- Event.Locked
- Event.LockedDate
- Event.Signed
- Event.SignedDate
- Sys_Forms:
- Study.Label
- Site.CountryName
- EventGroup.Label
- EventGroup.ExternalID
- Event.Label
- Event.ExternalID
- Form.Label
- Form.FirstSubmissionDate
- Form.SDVOverridePlan
- Form.SDVCompleted
- Form.FirstSDVDate
- Form.SDVLastModifiedDate
- Form.DMROverridePLan
- Form.DMRCompleted
- Form.FirstDMRDate
- Form.DMRLastModifiedDate
- Sys_ILB:
- Study.Label
- Site.CountryName
- EventGroup.Label
- EventGroup.ExternalID
- Event.Label
- Event.ExternalID
- Form.Label
- Form.ExternalID
- ItemGroup.Label
- ItemGroup.ExternalID
- Item.Label
- Item.ExternalID
- LABANALYTENAME
Enablement & Configuration
These changes apply automatically.
Learn More
Enhanced Query Listings Filters
Use Case
Improved filter options makes it easier for a user to select the data they wish to view.
Description
Query Listings in Clinical Reporting and CDB Workbench now support data type aware filters:
- Created on and modified on columns display a calendar in the filter
- Query status will display a picklist of status options
Enablement & Configuration
This feature is enabled automatically.
Export Enhancements
Use Case
Additional export options provide flexibility in determining which export type is best for your downstream systems.
Description
Exports from CDB Workbench and Clinical Reporting have been updated with the following new features:
- New columns have been added to listings of the Raw export type to show the decoded value for codelist items.
- System (SYS) Listings can now be added to an export of type None.
- A new group has been added to the Select Listings shuttle for SYS listings under Raw export types. If these listings are removed from the definition, they will appear as Available Listings.
Enablement & Configuration
This feature is automatically available to Export Listings. Existing scheduled exports of Raw type will not have the new decode columns included, these will only be added to new or modified export definitions. Existing None or Raw type scheduled exports will need to be updated to include or remove any System Listings.
Learn More
Study File Format API
Use Case
SFF allows users to keep their downstream systems in sync with data that is available through 15 minute incremental exports of EDC data.
Description
The Study File Format (SFF) export option provides data in an easy to consume format for downstream systems. It includes a manifest file that defines the data and a series of CSV files. SFF takes advantage of the Vault Direct Data API, allowing access to incremental packages if licensed.
The SFF API Access permission provides access to the new SFF API endpoints generated by CDB. By default, this permission is assigned to the CDMS Super User role.
Enablement & Configuration
Enable SFF by Study in EDC Tools. This feature is only available to production environments. Incremental data export requires a special license, contact Veeva Support for more information.
Learn More
Strict Manifest for 3PD & Open EDC
Use Case
This feature ensures that only defined data is ingested into CDB.
Description
A new optional parameter in the manifest .json file and manifest builder allow customers and third party data providers to restrict data ingested into CDB to only those items that are defined in the manifest. Previously, any items not defined were ingested as text fields, and all fields were ingested when included in the CSV file. Using a strict manifest setting for either the entire package, or for individual CSV files included in the package, the system will restrict the columns for import to only those defined. File level settings will override the package level setting for strict manifest.
Enablement & Configuration
This feature is automatically available, and it does not require any changes to existing manifest files unless users want to incorporate the strict manifest concept.
Date Added to Dashboard Refresh Time
Use Case
Adding the date ensures that if something has not refreshed for a long period of time, it is clear to the end user that a support investigation is needed.
Description
In the Workbench Review Dashboard and Clean Patient Tracker, the date has been added to the refresh time, to better show when the data was refreshed.
Enablement & Configuration
This feature is automatically available.
Disable Jobs for Inactive Studies
Use Case
Turning off system jobs ensures responsible use of resources, so the vault is not wasting energy processing data for studies that are no longer being used.
Description
When incremental import is disabled for a Study, the Study will be made Inactive (formerly known as Archived) in CDB. Studies can also be inactivated from the Studies list. Jobs that refresh Review Listings, the Clean Patient Tracker, or process the Direct Data API package for the Study will be stopped with inactivation.
Enablement & Configuration
This feature is automatically available.
Third Party Data Import Enhancements
Use Case
Additional datetime formats support external system output that no longer needs to be transformed before being ingested into CDB.
Description
Third party data import now supports these additional date time formats:
| Format | Examples |
|---|---|
| ddMMyyyy'T'HH:mm:ss | 10122024T16:15:30 |
| yyyyMMdd HH:mm:ss | 20241210 16:15:30 |
| MM/dd/yyyy HH:mm:ss | 12/10/2024 16:15:30 |
| yyyy-MM-dd'T'HH:mm:ss | 2024-12-10T16:15:30 |
Rejected third party import packages will now display in the audit log.
Enablement & Configuration
This feature is enabled automatically.
CDB API
The following are new features for the CDB API. See our Developer Portal's release notes for more detailed feature information.
CDB API Features
This release includes the following features for CDB developers:
- CDB Query Source
- Batch Limit for Open Query on Item API
- Return Individual Failures for CDB API Calls
EDC Migrator
Features in this section are new features for Veeva EDC Migrator.
Migration Vault: YAML Builder Enhancements
Use Case
These enhancements increase efficiency by automating tasks that used to be manually created.
Description
When a migration user initiates the YAML Builder job, Migration Vault will now:
- Create a new transformer selector for submitting Forms and Events with Visit Methods
- Create a new transformer selector for Events with Visit Methods
- Insert mappings for Lab Panel data within the form YAML
- Create YAML files for the study’s Event Groups and Events
- Create YAML files specific to Studies designed in Studio
- Create a YAML template for Events marked as Did Not Occur (DNO)
Enablement & Configuration
These enhancements are immediately available.
Learn More
Applying Frozen & Locked Statuses to Event Dates & Visit Methods
Use Case
This enhancement provides support for Visit Method within Migration Vault.
Description
In order to support the migration of visit methods, Migration Vault now allows configuration specialists to apply the Frozen and Locked statuses to Event Dates and Visit Methods.
The “attributes.csv” file now has a “field” column, which configuration specialists can use to indicate if the status should be applied to an Event Date (event_date) or Visit Method (visit_method). This field should be left blank if the attribute should be applied to both the Event Date and Visit Method. These statuses are now also applied when an ancestor object, such as a casebook, has the status applied.
Enablement & Configuration
This feature is immediately available.
Learn More
Migration Vault: Migrate Closed Queries
Use Case
Previously, any queries in the closed status were reopened upon ingestion, requiring users to review and reclose them.
Description
With this release, Migration Vault has been updated to ingest and migrate closed queries into CDMS. Upon ingestion, these queries remain in the closed state.
Enablement & Configuration
This feature is immediately available.