What's New in 22R3
Pre-Release Date: November 7, 2022 | CDB Pre-Release Date: November 14, 2022 | Release Date: November 18 & December 2, 2022We are pleased to bring you Veeva Clinical Data in 22R3. Read about the new features below. You can find information on enabling new features in the 22R3 Feature Enablement Details. Information on developer features (REST API) is in the Developer Portal.
Data Entry
Features in this section are changes to the Data Entry tab, a working area for investigators and clinical research coordinators to enter study execution data.
Bulk Casebook Signature
Use Case
Principal Investigators can sign multiple casebooks at once rather than signing each individual casebook at a time.
Description
This feature allows Principal Investigators to sign multiple casebooks at a time for a site. This feature also introduces the Signature History page in Data Entry, where users can view the signature history for all signing activities, both bulk and non-bulk. This page is available regardless of whether bulk casebook signature is enabled in a vault.
Enablement & Configuration
Contact Veeva Support to enable this feature. Once enabled at the Vault level, a Lead Data Manager must enable it for a study from EDC Tools then for individual sites in EDC Tools > Sites.
Default Settings for Data Entry
Use Case
There is more room on the screen for data entry, especially for users with smaller window sizes.
Description
The schedule tree in Data Entry is now responsive to the user’s browser window, devoting more space on the screen to data entry. The width of the schedule tree will still be customizable but will take up one-third of the Data Entry screen by default. If the user chooses to customize the schedule tree width, the selected width will stick across logged in sessions. In addition, the default height of the Common Log section is higher than previous releases to provide the user with better visibility.
Enablement & Configuration
This feature is available automatically.
Show the Delimiter for Composite Items in Data Entry
Use Case
Item labels will now be consistent for composite items when the form is blank or in Edit or View mode.
Description
The / Delimiter will now always be visible in the label for composite items in both Edit and View modes of Data Entry.
Enablement & Configuration
This change applies automatically.
Sign with Open Queries
Use Case
There can be times during the lifecycle of a study when all existing forms and event dates need to be signed regardless of whether or not there are open queries. This feature allows those forms and event dates to be signed when necessary.
Description
Principal Investigators can apply their signatures to forms and event dates that have open queries.
Enablement & Configuration
Contact Veeva Support to enable this feature.
Data Review
Features in this section are changes to the Review tab, a working area for clinical research associates and data managers, or to review functionality within the Data Entry tab.
Ability to Specify Other Reason for Additive Review
Use Case
This feature provides flexibility for users performing Additive Review.
Description
When the “Enable Other Specify” reason for change and “Enable Additive Review” option are both set to “Yes” in Studio, CRAs and Data Managers can provide a custom reason for Additive Review.
Enablement & Configuration
This feature is automatically enabled for Studies where Additive Review is enabled.
Protocol Deviations Enhancements
Description
With this release, we have made various enhancements to Protocol Deviations which are detailed below.
- We have added a new column, Protocol Deviation ID, which will display in the Protocol Deviation grid and in both exports. This column allows users to find a deviation in the export by its unique ID number.
- We have made improvements to the Protocol Deviation search, so that users will now be able to search on the Protocol Deviation ID or Summary. In addition, the search is more flexible and case insensitive, so users no longer need to enter an exact match.
- When viewing a Protocol Deviation in the Review UI, users will now be able to see the deviation’s status in the dialog. Users will only see active Protocol Deviations, such as those with an Open or Investigating status. Inactive Protocol Deviations can only be viewed in the Protocol Deviation grid.
Enablement & Configuration
These enhancements are automatically available in Studies using Protocol Deviations.
Protocol Deviations: Export as an Excel™ File
Description
Protocol Deviations can now be exported as an Excel™ file. The Date fields in both the CSV and Excel™ exports also have been updated to ISO format (YYYY-MM-DD). In addition, users can now export a filtered list of deviations.
Enablement & Configuration
This feature are automatically available in Studies using Protocol Deviations.
Clinical Coding
The following are new features for Veeva Coder, the clinical coding area for Veeva Coder.
Coder UI Enhancements
Description
We have made the following UI enhancements to Coder with this release:
- Collapsible Properties Panel: users can collapse and expand the Properties Panel as necessary while coding
- Optimized white space: the Verbatims table will now occupy available white space to display more Verbatims
Enablement & Configuration
This feature is enabled automatically.
Coders are Allowed to Code a Form with a "Needs Synonym List" Status
Use Case
This feature allows Coder more freedom to complete their tasks while Coder Administrators are administering Synonym Lists.
Description
If a Form does not have an assigned Synonym List then the Coder may continue to code the Form until one is assigned. Forms with the Needs Synonym List status will not Autocode or have Suggestions off of the Synonym List.
Enablement & Configuration
This change applies automatically.
New Upversioning Status in Coder
Use Case
Users can easily identify which Forms and Synonym Lists failed Upversioning so that they can restart Upversioning on these Forms and Synonym Lists.
Description
With this release, we’ve introduced a new status called Failed Upversioning that will display when Upversioning fails for a Form or Synonym List. This status allows Coder Administrators to easily identify which Forms and Synonym Lists failed Upversioning. The Upversioning job has also been improved so that Coder can upversion more code requests in less time. As part of this change, the code request object no longer maintains the Dictionary Release field. To reference Dictionary Release data in Vault Reports, view the Dictionary Release field value from the Medical Coding Item Definition object.
The Failed Upversioning status is visible to Coders on the Coder home page and Verbatims page. Vault will notify users when a Form has the Failed Upversioning status. Users can continue to code the Form until the Coder Administrator restarts Upversioning.
Synonym Lists in this status cannot not be assigned to any Forms until a Coder Administrator restarts Upversioning and the upversion succeeds.
Enablement & Configuration
Auto-on.
Study Design & Configuration
Features in this area apply to Studio, the study design and configuration area for Veeva EDC.
Cross-Form Derivations
Use Case
The traditional challenge that cross-form calculations pose on EDC systems is that changing data on one form that is used to derive values onto another requires manual action from the user to the form with the resulting value. This feature provides a way to carry forward values from one form to another while displaying accurate, updated data even on submitted and non-materialized forms.
Description
With this release, we’ve added the ability to derive values across forms in a casebook. Study Designers can add the new “Derived Items” component to forms and create Set Derived Value rules to set values of derived items based on criteria across a casebook.
Derived items differ from traditional item types in that derived item values don’t depend on the submission of a form, and derived values can be updated outside of the constraints of traditional Data Entry (such as form submission, study locking and freezing, and SDV/DMR). Derived items can be configured to display or not display in both Data Entry and extracts.
EDC Tools users can now run Set Derived Values rules as part of the Run Rules job in Tools > EDC Tools > Rules.
Enablement & Configuration
The Derivation Version must be set to “Cross Form” on the study’s Study Configuration record.
Rules: Referencing the Previous Value of an Object with @PreviousEvent
Use Case
This feature reduces the need for custom triggers. It also provides study designers with more flexibility when designing query rules and reduces the need for CRAs and data managers to perform manual checks on blank data.
Description
Query rules can now locate previous values for Events, Forms, and Items in a Casebook, given the context of the current Form being submitted and its parent Event. The previous Event is determined using the chronological order of Events in the schedule.
Values that occur throughout a Study but don’t necessarily repeat or follow a set pattern often need to be compared to a value in the previous iteration of that Form, or to another value in the previous Event. This feature allows data to be referenced based on the chronological order in which a subject progresses through visits. For example, a rule can now compare a subject’s dosing record at a given Event with their dosing record from the Previous Event. When event dates change, or occur out of chronological order, or when Unscheduled Events are inserted between scheduled study visits, @PreviousEvent rules will identify such changes and be re-evaluated in the context of a casebook’s current event date order.
The identifier @PreviousEvent must be used along with @Form identifiers, which are required to give context to determine the previous value. @PreviousEvent rules are executed as post-save rules.
As part of this enhancement, for rules using the [-1] and [+1] functionality to determine the previous or next value of a repeating object, users can now configure whether the rule should skip over values that have been marked as Intentionally Left Blank.
Enablement & Configuration
This feature is automatically available to Studies using Expression Engine V2.
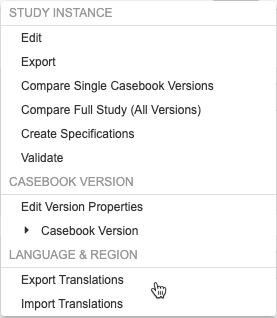
Multi-Language Support in Studio
Use Case
While the ability to import translations was possible through Vault Platform translation features, this feature simplifies that process by allowing Studio users to import and export their study and version specific Labels for their definitions.
Description
Studio users can now import and export translated study labels and messages for use in a translation workbench. Users can choose one or more languages to export, send those to a translation service, and then import the translations. Studio can process multiple languages at once, and each language goes into its own tab-delimited CSV file. While the file format is CSV, the files are tab delimited, as study labels can contain commas. The Export Translations and Import Translations actions are available in the new Language & Region section of the Studio Actions menu.
Translations must be done as part of the study’s build process and prior to deploying to production.
This release doesn’t include translations for vault-level labels for Lab Analytes or custom Reasons for Change and Intentionally Left Blank Reasons.
Enablement & Configuration
This feature is available automatically in Studio. This functionality was previously only available in Admin > Settings.
Learn More
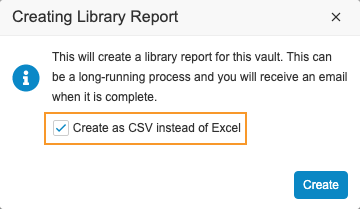
Create Library Report as CSV
Use Case
Excel™ has a million record limit. Having the report in a tab-delimited format allows for the processing of the Library Report in other tools, such as Microsoft Access™.
Description
With this release, librarians can now create the Library Report in tab-delimited format (with a .csv extension), instead of an Excel™ file. Vault automatically uses this format if there are more than 800,000 records in the Definition Histories object and will not give users the choice to export as an Excel™ file.
Enablement & Configuration
This feature is available automatically to users with permission to create a library report.
Form and Item Linking Rules Allow References to Fully-qualified Identifiers
Use Case
This enhancement allows more data to be accessed by a single rule, reducing the need for custom triggers.
Description
With this release, rules containing Form and Item links now allow references to fully-qualified identifiers outside of the linked identifiers. Linking Rules that contain fully-qualified outside identifiers will automatically be required for execution as a post-save rule.
Enablement & Configuration
This feature is automatically available for Studies using Expression Engine V2.
Option to Include Keys & Origin in SDS
Use Case
This feature provides users with an easy way to retrieve details about the origins of objects in their Study, as well as tie-ins to external systems using Veeva-managed keys. For example, the Origin Key (origin’s Cross Vault Unique ID) could be used to tie that definition to the metadata repository.
Description
Studio users can choose to include additional key values in their SDS to use for troubleshooting or tracking definitions across other applications, such as a metadata repository.
The available options include:
- Include Keys: This option adds columns for Cross Vault Unique ID and Private Key. The Cross Vault Unique ID provides the vault, study, and record IDs and is unique to the version of the object. The Private Key is used to define the object across versions and studies.
- Include Origin: This adds columns for origin keys. This set of keys tie the object to its point of origin, typically the library. These include the Origin Name (name of the study or library collection), Origin Type (either Study or Library), the Origin Definition Name (the current Name of the object in the origin study or library), and the Origin Key (the Cross Vault Unique ID tying the object to the Origin).
Enablement & Configuration
This feature is auto-on. Studies created prior to the 21R1 release (initial release of the Library) may have missing origin records. Studies created after the 21R1 release have all origin records.
Show Codelist Labels in Email Notifications
Description
Send Email rules that contain references to codelist items now display the codelist label in addition to the backend coded value. Values are now displayed in the subject line and email body as “CODED VALUE(Label Value)”.
Enablement & Configuration
This change applies automatically.
Automatic Removal of Invalid Event Window Configuration for First Event
Use Case
This removes labor to fix a configuration that wasn’t available for the newly moved Event.
Description
This includes minor enhancements to the schedule design in Studio. When the user moves an existing Event as the first event in the schedule the system will now automatically remove the invalid window reference.
Enablement & Configuration
This feature is auto-on and available for all studies.
Unit Item Enhancements
Description
With this release, the error message for Unit Items has been updated for clarity and consistency with other number fields. Negative signs will no longer count as characters in a Unit Item. The default length and precision for unit items has been updated to 14 and 0.
When an item is linked with a form with display items that are units or global lab units, the units will display in Detail PDFs, Closeout PDFs, and Assessment PDFs.
Enablement & Configuration
These enhancements are available automatically.
Study Administration
Features in this section apply to System Tools or EDC Tools, a study-level administration area for Veeva EDC.
Principal Investigator No Longer Required for Site Creation
Description
The Site Principal Investigator field is no longer required when creating a new Site.
Enablement & Configuration
These enhancements are available automatically.
Events Support Event Window
Use Case
With this change, the Event Window Status and Days Out of Window will calculate based on the new Event Window Definition.
Description
The system will now calculate if an event is “Out of Window,” and if so, how many days out of window.
Enablement & Configuration
This feature is available automatically.
Include Freeze, Lock & Sign Dates in the Form Progress Listing for DM1 Studies
Use Case
This increases consistency with Data Model 2 studies.
Description
For studies on Data Model 1, the Form Progress Listing will now include values in the following columns:
- Freeze Date
- Lock Date
- Sign Date
These columns were previously only populated for Data Model 2 studies.
Enablement & Configuration
This change applies automatically in Studies using Data Model V1.
Include Restricted Forms and Events in Counts for Subject Progress and Event Progress Listings
Use Case
With this feature, count columns will respect the restricted job option.
Description
If the user selects Include Restricted Data in the Subject Progress Listing or Event Progress Listing job dialog, the job will include restricted forms and events in columns that count the number of forms and events. If “Include Restricted Data” isn’t selected, the listings won’t include restricted forms and events.
Enablement & Configuration
This feature is automatically available.
Job & Rule Execution Enhancements
Use Case
Users also have the ability to delineate between jobs that have been initiated but have not begun processing and are shown a completion percentage and timestamp for the last job fragment activity.
Description
With this release, we have removed the casebook limitation in the Run Rules job and doubled the amount of rules that can be run within one job. In addition, we have updated the EDC job retention policy to 30 days.
Enablement & Configuration
These changes apply automatically for all bulk EDC jobs.
New Column for All Items in the Form Progress Listing
Use Case
This feature allows users to determine how many items on the form have data, compared to the total number of items.
Description
A new column for All Items will now be available in the Form Progress Listing. This column will be included in the listing when “Include Item Counts” is selected in the job options dialog. The purpose of this column is to count the total number of items on the form, allowing users to compare this value with the number of items with data and the number of items with data change.
Enablement & Configuration
This feature is automatically enabled for Data Model 2 studies.
Notify Users when a Site is Locked from Review Plan Assignment
Description
With this release, Vault notifies users that a site is locked from EDC Tools > Review Plan Assignment.
Enablement & Configuration
These enhancements are available automatically.
Show Progress Percentage for In Progress Jobs
Use Case
Users also have the ability to delineate between jobs that have been initiated but have not begun processing and are shown a completion percentage and timestamp for the last job fragment activity.
Description
Users can see the progress completion percentage of In Progress jobs as the job runs.
Enablement & Configuration
These changes apply automatically for all bulk EDC jobs.
Include User Role in Query Detail Listings for Studies Not Using Query Teams
Use Case
Users can identify the roles that are creating, answering and closing queries, even if the Query Teams feature is not enabled.
Description
When a study isn’t using the Query Teams feature, Vault now populates the following Query Detail Listing columns with the user’s current study role: Created By Role, Answered By Role, and Closed By Role.
Enablement & Configuration
This feature is automatically available.
SDE Enhancements
Use Case
These enhancements support cross-functional features and add more information to SDE datasets.
Description
The following changes have been made to the 22R3 version of the SDE:
- The SYS_PD dataset will include Category, Subcategory, and Severity Labels along with Form Sequence, Form VOF ID, and Protocol Deviation VOF ID.
Enablement & Configuration
These enhancements are auto-on for the 22R3 version of the Study Data Extract job.
SDE: Include Study Design Metadata
Use Case
Users will be able to use study design information in tandem with clinical data for their downstream analysis.
Description
The 22R3 version of the SDE will have the option to “Include Study Design” in the New Job dialog. When checked, Vault will add a “definitions” folder to the zip package which contains CSVs with study design information.
Enablement & Configuration
This feature is automatically available for the 22R3 version of the Study Data Extract job.
SDE: Length Standardization
Use Case
Standardizing text column lengths across the SDE will prevent data value truncation.
Description
In the 22R3 version of the SDE, instead of defaulting SAS text column lengths, Vault will use Vault and Studio configuration data to determine column lengths.
For 22R3, codelist Decodes (Labels) will use 256 characters for length and 1024 bytes as the column length. In 22R2 and earlier versions, these will use 1500 characters for length and 1500 bytes as the column length.
Enablement & Configuration
This feature is automatically available for the 22R3 version of the Study Data Extract job.
Select Sites Across Multiple Pages when Running a Retrospective Amendment
Description
Users can select multiple Sites across pages when selecting Sites for a Retrospective Amendment.
Enablement & Configuration
These enhancements are available automatically.
Include Site Number in Study Summary Metrics Report
Use Case
Users can now identify a site by its number or its name.
Description
The Study Summary Metrics Report now includes a column for Site Number.
Enablement & Configuration
This feature is automatically available.
Study Progress Listings in Reports
Use Case
This feature provides the ability to customize and filter Study Progress Listings.
Description
Study Progress Listings are now available in the Reports tab. To populate the reports with data, a user will need to schedule a listing job in EDC Tools and select the option to send the data to Vault Reports. Veeva recommends scheduling this listing as a daily job to ensure that the latest data is available in the reports.
As part of the job, Vault sends the listing data to the appropriate report. In the Reports tab, there are standard templates that match the output of the listing from EDC Tools. Users with access to Reports can create their own version of the report based on the standard template, which they can customize by adding filters or by adding or removing columns.
We added the following objects to support this feature:
- Event Progress Listing (
event_progress_listing__v) - Form Progress Listing (
form_progress_listing__v) - Query Detail Listing (
query_detail_listing__v) - Subject Progress Listing (
subject_progress_listing__v)
Enablement & Configuration
Contact Veeva Support to enable this feature in your vault.
Labs
Features in this section are new features for the Labs module of Veeva EDC.
Labs: Configurable Queries
Use Case
This feature provides more control and flexibility for Sponsors and CRO’s over system-generated Lab queries.
Description
With this release, users can enable or disable the following system-generated Lab queries:
- Lab Units Do not Match
- Lab Age Calculation
- Missing Lab Results
Enablement & Configuration
This feature is available automatically in Studies using Labs.
Labs: Support for Lab Results with Characters '>' and '<'
Use Case
This feature provides additional support for lab results that are more or less than numbers or units.
Description
With this feature, users can use “>” and “<” characters when entering Lab results in Data Entry.
Enablement & Configuration
This feature is automatically enabled in Studies using Data Model V2 and Global Versionless Labs.
Labs: Ability to Disable Pending Labs
Use Case
This feature provides flexibility by allowing or not allowing Sites to add new Lab Locations.
Description
With this release, users can configure whether or not Sites can enter new lab locations and associated lab normals.
Enablement & Configuration
This feature is available automatically in Studies using Labs.
Labs: Global Lab Units & Codelists Display in UI Grids
Use Case
This feature provides consistency with other data entry grids.
Description
For studies using Global Labs, the Lab Units and Codelists will display in the following grids:
- Repeating Form Grid
- Form Linking Grid
- Item Linking Grid
Enablement & Configuration
This feature is available automatically in Studies using Labs.
Role Management & Security
Features in this section are enhancements to the System Tools > Role Management and System Tools > Users areas, as well as changes to standard Study Roles, security, and access control in Veeva Clinical Data.
User Import Enhancements
Description
With this release, we have introduced a number of enhancements to manage Users and Roles, including the following:
- Users will be warned when editing an existing user’s details when importing
- Users will be warned when overlapping site and country access is specified when importing users
Enablement & Configuration
These enhancements are available automatically.
User Training Report Includes Users with All Sites Access
Description
The User Training Report now includes Users with All Sites access.
Enablement & Configuration
These enhancements are available automatically.
Tooltip for Add as PI Field
Description
In System Tools > Users, the Add as PI field now includes an explanatory tooltip.
Enablement & Configuration
These enhancements are available automatically.
Deployments
Features in this section are enhancements to deployment functionality in Veeva Clinical Data.
Vault Diff Report & Multi-Role Security
Use Case
With the introduction of Multi-Role Security, the Vault Diff Report’s User Defined Roles section does not accurately reflect custom roles, instead displaying differences in the Security Profiles which is less applicable with the MRS model. This feature restores the ability of the Diff Report to provide accurate role and permission information regardless of the security model.
Description
Deployment Administrators can now run the Vault Diff Comparison Report and see details of roles and permission differences between Vaults, whether those Vaults are using Multi-Role Security or not.
Enablement & Configuration
This feature is auto-on. Customers using Multi-Role Security in all of their Vaults will immediately see their custom role and permission differences listed in the report. Customer’s not yet using Multi-Role will see the same Vault Diff Report as they currently do.
Disallow Destructive Changes
Use Case
This is to avoid the deletion of any data in production due to an accidental change in the casebook definition.
Description
This feature provides a hard stop for destructive changes in Studies that have data, while still providing the ability to make these destructive changes in Studies where there isn’t data yet (for example, a split go-live).
Destructive changes include any of the following:
- Removing an Event Group from the casebook schedule
- Removing an Event from an Event Group
- Removing a Form from an Event
- Removing an Item Group from a Form
- Removing an Item from an Item Group
With this feature, we added clearer warnings in Studio when a study designer makes one of these destructive changes. We also updated dialogs for amendments and deployment to include a warning when a version contains a destructive change.
As a workaround, we recommend using dynamic rules to remove components from the schedule.
If a destructive change is blocked by the retrospective amendment, and that amendment needs to be applied, an organization can contact Veeva Support and request that the amendment be allowed.
Enablement & Configuration
This feature is enabled automatically and applies to all Studies.
Retrospective Amendment Start Event Requires the First Event
Use Case
This change is to avoid issues with mixed-version Casebooks (where a Subject has Event Groups with their Events and Forms on different versions).
Description
This feature removes the ability to select the Start Event for a retrospective amendment. Now, Vault automatically uses the first Event in the first Event Group on the casebook schedule. Vault now only permits retrospective amendments on the entire Casebook starting from the first Event Group and Event.
Enablement & Configuration
This change applies automatically to all Studies.
Connections & Integrations
Features in this section are new connections or integrations with Veeva Clinical Data or enhancements to existing ones.
E2B Link: Configuration Deployment
Use Case
Now that the DEV environment is the only environment that allows configurations, organizations will enjoy a controlled and uniform configuration across their study environments. This allows for transparency in what is available in the E2B Link Safety Configuration during the UAT and production phases of the Study.
Description
E2B Link’s Safety Configurations now must be configured in a development (DEV) study environment and then deployed to downstream UAT and production environments.
During deployment, the provided validation file now includes validation checks of the Safety Configuration. If there is a situation in the configuration that would prevent the E2B XML from being generated or from including expected relevant case data, then that will show up in the validation file as either an error or a warning.
Enablement & Configuration
This feature is available automatically.
E2BLink: Email Notifications
Use Case
Users can receive notifications when a Safety transmission fails and even prior to it failing so that the connection administrator can take action.
Description
E2BLink captures up to five email addresses to send email notifications when the transmission fails via the Safety to CDMS Connector or via the AS2 Gateway to a third party Safety System. It will also send an email prior to the expiration of the AS2 Gateway Encryption Certificates.
Vault can now send email notifications when a transmission fails, there is an expired certificate, or there is an inactive connection or gateway profile. Vault also can send a notification when the AS2 Gateway Encryption Certificate is about to expire. Vault sends the notification three months prior, one month prior, and the day the certificates expire, giving your team enough time to respond and load new certificates. Administrators can specify up to five (5) email addresses to receive these notifications from Tools > System Tools > External Connections.
As part of this feature, we changed some UI labels in EDC Tools > Safety Configuration:
| Original Label | New Label |
|---|---|
| Safety System | Safety System Connection Profile |
| Send Adverse Events to Safety System | Study Transmission Status |
| Treatment | Study Drug |
| Include Treatment Form | Include Study Drug Form |
| Treatment Form | Study Drug Form |
| Treatment Name | Study Drug Name |
| Item Indicating Treatment Administered | Item Indicating Study Drug Administered |
| Item Value Indicating Treatment Administered | Item Value Indicating Study Drug Administered |
| Add Treatment | Add Study Drug |
Enablement & Configuration
This feature is available automatically.
Clinical DataBase (CDB) & EDC Clinical Reporting
The following are new features for the Veeva CDB application, EDC Clinical Reporting (the Veeva Clinical Data solution for data cleaning and reporting), or both.
Availability: Clinical DataBase (CDB) is only available to CDB license holders. Contact your Veeva Services representative for details.
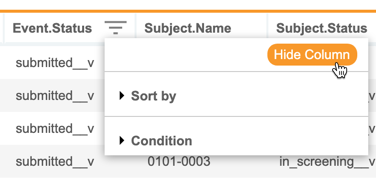
Hide Columns in Listings, Checks & Views
Use Case
This feature provides a better review experience for data managers with smaller displays.
Description
With this release, CDB users can now hide and show columns in all core and custom listings, checks, and views. The Sort & Filter menu now has a Hide Column option. When a column is hidden, CDB displays an orange, dotted line in place of the column. Once a user hides at least one column, CDB shows the Unhide Columns button, which includes a count of how many columns are hidden.
As part of this enhancement, users no longer need to click OK to apply a sort order from the Sort & Filter menu.
Enablement & Configuration
This feature is enabled automatically to all users.
Multiple Schedules per Export Definition
Use Case
CDB users can have greater flexibility when scheduling exports.
Description
CDB users can now have multiple scheduled exports per Export Definition. The Properties panel includes an Add Schedule button when there is an existing schedule.
Enablement & Configuration
This feature is enabled automatically to all users who can create and modify Export Definitions.
Reference Objects
Use Case
Reference objects address the need to define non-clinical data during the import process, so that the data can be used alongside clinical data for cleaning and export purposes.
Description
CQL now supports reference objects. Reference objects are like Forms, in that they contain a limited set of Items, but they are different in that a reference object must define how it relates to other header or form data. This relationship is defined upon ingestion. Once the data has been defined and ingested, CQL can use it like any other Form.
Reference objects are defined in the manifest file for an import package. Then, the data for the reference object is imported as a CSV file in that import package.
This feature introduces a new CQL function, KEYMATCH, which is only available for use with reference objects. The KEYMATCH function checks if the related_item matches the related_key.
Enablement & Configuration
The Reference Objects feature is enabled by default, but users must define reference objects to use them.
Automated Checks Re-query
Use Case
Organizations can automate re-queries.
Description
After a check opens a query against a record, and that query is closed, if a data change occurs such that the record reappears in the check, CDB re-queries the Item or Event Date.
Enablement & Configuration
This feature is available automatically. Automated checks begin re-querying automatically, with no additional configuration required.
CDB Data Provider Query Access
Use Case
Organizations can now allow data providers access to a given data source to answer queries.
Description
Administrators can now define a user’s source access (for data providers) to answering queries within CDB > Admin > Users. Once given access, data providers can access select listings in CDB to answer queries associated with their specific data source.
This feature adds the following new permissions:
Assigned to the CDB Data Provider, CDMS Super User, and CDMS Data Manager study roles:
- View Selected Listings
- View Selected Query Listings
- Answer 3rd Party Queries
Assigned to the CDMS Super User, CDMS Data Manager, and CDMS Lead Data Manager study roles:
- View All Listings
- View All CDB Query Listings
Enablement & Configuration
This feature is available automatically. Vault Administrators can set user access from Admin > Users.
Learn More
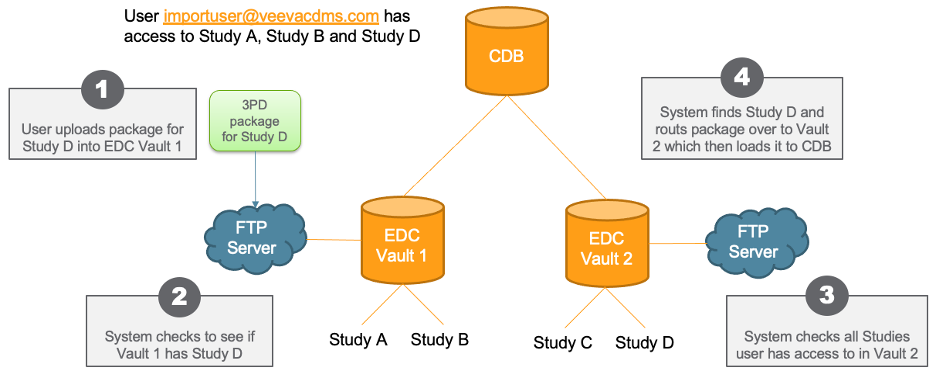
Route 3rd Party Packages to the Right Vault in the Domain
Use Case
This feature allows for easier management of FTP credentials when uploading data to CDB.
Description
For organizations with more than one vaults in their domain, their credentials are different for each vault because the username contains the the vault’s DNS and the Vault User Name, for example, “prod1-pharmacdms.veevavault.com+tsmith@veepharm.com”. Because a customer or data vendor may not know exactly which vault a Study resides in, they may not know which user name to use when uploading their package to the FTP server.
To minimize confusion, CDB now automatically checks all vaults that a user can access for the Study referenced in their package. If CDB finds the Study in the package in the current vault, then CDB loads the package as it does today. If the current vault doesn’t contain the Study, CDB checks all other vaults the user can access. When it finds the vault containing the Study, CDB loads the package into that vault. Users must be able to access the FTP server in the appropriate vault to load the Study.
Enablement & Configuration
This feature is enabled automatically.
Support for Queries on CDB Derived Fields
Use Case
Users no longer need to access the source Item for a derived field to open or close queries against the data point.
Description
Users can now view the cell details for a CDB univariate, derived field. From the Cell Details panel, they can now open and close queries on the underlying EDC or third party data Item.
Enablement & Configuration
This feature is enabled automatically to all users.
3rd Party Data Load Error Rollback
Use Case
During the course of a study, if a vendor loads a bad package, the study isn’t blocked from ingesting data from other vendors. This ensures that the cleaning of data from other sources can continue and that an errored source won’t result in a bottleneck.
Description
With this release, if the import of a third party data package fails, CDB now automatically rolls back to the last successfully loaded package for that Source. Prior to this release, if a load failed, all new data from any source was blocked until the error was corrected.
If there isn’t a successful package for the source to rollback to, then no new data is imported from any source until the error is fixed.
Enablement & Configuration
This feature is enabled automatically.
Reduce Header & Unit SAS Lengths in CDB Exports
Use Case
By reducing the SAS lengths of text column headers, users can save space when allocating memory in their downstream systems to ingest export data.
Description
CDB now reduces the SAS lengths of column headers in exports. Prior to this release, the length limit was defaulted to 6000 bytes. This change applies to column headers in core and system listings, as well column headers for all unit columns.
The following @HDR, @Form, @ItemGroup, and Item property columns were reduced from 6000 to 512 bytes:
- @HDR.Study.Name
- @HDR.Site.Name
- @HDR.Site.Number
- @HDR.Site.Country
- @HDR.Site.PI
- @HDR.Subject.Name
- @HDR.Subject.Status
- @HDR.EventGroup.Name
- @HDR.Event.Name
- @HDR.Event.Date
- @HDR.Event.Status
- @Form.Name
- @Form.Status
- @Form.ExternalID
- @ItemGroup.Name
- Name (on the Item)
The following ILB reason property columns were reduced from 6000 to 1020 bytes:
- @Form.ILBReason
- ILBReason (on the Item)
The following unit columns were reduced from 6000 to 512 bytes:
- Item_UOM
- Item_UOM_TRANSLATED
Enablement & Configuration
This change applies automatically to all exports.
CDB Support for Item to Form Linking
Use Case
Users can create custom listings that include Item to Form Links using CQL.
Description
CDB now supports Item to Form Linking by using the newly defined @FormLink notation.
Enablement & Configuration
This feature is enabled automatically.
EDC Migrator
Features in this section are new features for Veeva EDC Migrator.
Veeva Migration Reports
Description
This release introduced Veeva Migration Reports, a collection of reports about the state of migrated objects after a Study has loaded into the staging database. Migration users can initiate report creation from within Migration Vault, using the Excel - Veeva Migration Reports action in the load’s Actions menu, and then Vault emails the reports to that user.
These reports are delivered as a single Excel™ file, with a separate worksheet for each report.
Veeva Migration Reports include the following reports:
- Failed_Skipped_Task_Types: Lists tasks (for example, CREATE_EVENT_GROUP or CREATE_FORM) that failed or were skipped with the reason why.
- Orphaned_Task_Types: Lists tasks (for example, CREATE_FORM or CREATE_ITEM_GROUP) that don’t have a corresponding parent.
- Repaired_Objects: Lists objects that were created by the repair process, as well as repaired objects for which attributes weren’t applied with a list of those attributes.
- Task_Types_With_Failed_Skipped_Parent: Lists tasks (for example, CREATE_ITEM GROUP or CREATE_ITEM) that haven’t been processed because their parent has a Failed or Skipped status.
- Task_Type_Status_Count: Displays task Status by count.
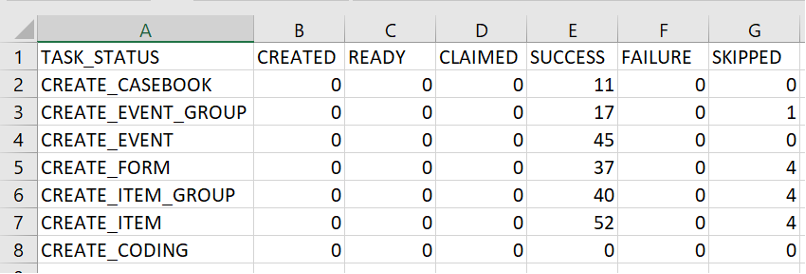
Enablement & Configuration
This feature is automatically available.
Configurable Identity Support
Description
In some Studies, a fully-qualified identity, or name, is not always available in the source data, but it is available through an external reference. With this feature, we replaced the current identity concept by using an external reference that can be derived from any source data values and creating a configurable “identity” independent of stating, pre-processing, and post-processing.
All objects use the new configurable identity. If an object, for example, a form, isn’t specifically configured in the YAML file, Migration Vault uses the default identity, as determined by the system.
To use this feature, users must configure their YAML mapping files to use any column or combination of columns to create identities that uniquely identify an object in Vault or in an external system. Users can configure identities at the Casebook, Event Group, Event, Form, Item Group, and Item levels.
The YAML below shows the default settings that are applied if a configurable identity is not provided in the header. This can also be explicitly placed in the header YAML to simulate the legacy identities.
identities:
casebook: [SUBJECTNUMBERSTR, SITEMNEMONIC]
eventGroup: [ $PARENT_REF, $VEEVA_DEF, $SEQUENCE ]
event:[ $PARENT_REF, $VEEVA_DEF ]
form: [ $PARENT_REF, $VEEVA_DEF, $SEQUENCE ]
itemGroup: [ $PARENT_REF, $VEEVA_DEF, $SEQUENCE ]
item: [ $PARENT_REF, $VEEVA_DEF, $COLUMN_NAME ]
query: [ $PARENT_REF, QUERY_ID ]
queryMessage: [ $PARENT_REF, MESSAGE_SEQUENCE ]
For the “queries.csv” and “attributes.csv”, the new REFERENCE and REFERENCE_TYPE columns are required, even if the Study isn’t using configurable identity. In that case, include the columns, but leave them blank.
Enablement & Configuration
This feature is automatically available.
Support for Migration of Code Requests with ATC4 Level Matches
Description
This cross-functional feature is a continuation of work initiated in 22R1. This feature supports WHODrug medical dictionary coding requests for ATC Codes 1 - 4. Previously, only ATC 4 Codes were supported. Prior to this release, coders would have to manually re-code the terms that did not match at the ATC4 level, which was a particularly time-consuming effort for larger studies.
Migration Vault utilizes the user-defined YAML in order to send an updated coding request to Vault Coder that can be matched against ATC Codes 1, 2, 3, or 4. The new YAML block must:
- Be named
atcCode - Contain a generic
columnNamethat accepts and supports any ATC Code, 1 through 4 - Contain a reference to the medical coding transformers
Enablement & Configuration
Users must update their YAML mapping to use this feature.