What's New in 21R2
Pre-Release Date: July 19, 2021 | Release Date: July 30 & August 6, 2021We are pleased to bring you Veeva Clinical Data in 21R2. Read about the new features below. You can find information on enabling new features in the 21R2 Feature Enablement Details. Information on developer features (REST API) is in the Developer Portal.
Clinical Data
Features in this section are changes that apply to all application areas of Veeva EDC and CDB.
Pop-Up Window Support for CDMS SSO Authentication
Use Case
The system now provides more flexibility in SAML Profile configurations for authentication occurring within Vault CDMS.
Description
Vault CDMS now supports pop-up windows for SSO authentication with SAML Profile configurations, in addition to the iFrame support introduced in 21R1. For Vaults configured with the pop-up window, users will authenticate with their credentials in a separate pop-up window, instead of within the current browser tab.
Customers using IE11 with SSO enabled must enable the SAML eSignature feature.
Randomization Users: Customers who use Randomization on IE11 with SSO must enable the SAML eSignatures feature to access certain Randomization features.
Enablement & Configuration
A Vault Owner must select the Authenticate SAML eSignatures in a pop-up window rather than an iFrame checkbox on the SAML Profile to enable the pop-up flow. SAML Profiles without this checkbox selected will continue to operate within an iFrame.
Data Entry
Features in this section are changes to the Data Entry tab, a working area for investigators and clinical research coordinators to enter study execution data.
EDC General Enhancements
Use Case
The View Summary dialog provides an easy way to view multiple repeating Form instances within a single view and easily jump to any given instance. Defaulting the unit value when only one exists allows for Data Entry units to be more efficient.
Description
With this release, we made the following general enhancements to the Data Entry and Review areas of Vault EDC:
- The Repeating Form Expansion feature is now automatically enabled for all studies.
- Review tab users can click View Summary for a repeating form in the Review tab’s Event & Form List panel to view a table that contains all instances of that Form. When viewing the summary, users can click into any row to scroll to that Form instance in the Content panel. This feature allows users to quickly navigate to a specific instance rather than scrolling up or down on the page.
- Prior to this release, repeating Forms were initially loaded in the selected Event in a grid view for the user to click into a specific instance of that Form. With this release, repeating Form Instances display in the same layout as non-repeating Forms. Each instance is loaded as the user scrolls through the Event. The initial grid view no longer displays.
- In Data Entry, if there is only one Unit Item available, Vault automatically defaults to that Unit Item, instead of requiring site users to select a Unit Item when there is only one.
Enablement & Configuration
The Repeating Form Expansion setting must be enabled for the Study. Unit defaulting will automatically apply to any study with a unit that only contains one option.
Editable Grids
Use Case
Editable Grids/Tabular Data Entry allows for site users to more efficiently enter data into repeating item groups.
Description
Study Designers now have the ability to configure a repeating item group to appear in Data Entry in an Editable Grid format. Editable Grids allow for site users to efficiently enter data in a tabular, spreadsheet-like, format. Tabular data entry enables the user to quickly enter and view data across multiple items and item group instances without the need to continuously scroll up and down the page. This updated format is also an easier way to enter data for users who are accustomed to interacting with spreadsheets.
Users can access the display format for repeating item groups in the properties panel for the selected Item Group in Studio. The Editable Grid display format, when enabled in Studio, will show the item group to the site user in Data Entry in a table format, with each item displayed in a separate column. The Editable Grid item group displays the tabular format for both submitted and unsubmitted forms.
Enablement & Configuration
Study Designers can configure any new or existing repeating item group in Studio via the Item Group Properties Panel. Editable Grids are only supported on Data Entry V2 studies.
Data Review
Features in this section are changes to the Review tab, a working area for clinical research associates and data managers, or to review functionality within the Data Entry tab.
Enhanced System Query Closure Restrictions
Use Case
Sponsors can now restrict which users can close queries that were created by the System.
Description
The Query Team Restrictions feature has been expanded with this release to include system queries. Previously, Query Teams could only be applied to manual queries. Now, study designers can choose to set the Query Team that is applied to each system query via Studio’s Settings page for the given Study. System queries that are tagged with a Query Team can only be closed by users in a role on that same team. For example, a study designer can choose to tag all system queries with the Data Management team. As the system creates queries on Forms, those queries will be tagged with the Data Management team. Only roles on the Data Management team (the standard CDMS Data Manager and CDMS Lead Data Manager study roles, and any custom Study Roles assigned to that team) will have the ability to close these queries. Vault disables the Close Query button for users outside the specified Query Team, with a message to inform them that they don’t have the ability to close this query.
Enablement & Configuration
Study designers can configure this feature from Studio > Settings when Enable Team Query Restrictions is set to Yes for the Study.
Clinical Coding
The following are new features for Veeva Coder, the clinical coding area for Veeva Coder.
JDrug
Use Case
Japanese language studies are more holistically supported in CDMS.
Description
Vault Coder now offers JDrug to code and autocode Japanese medications.
To code in Japanese, Vault Coder searches the Dictionary using several Japanese scripts, specifically Kanji, Hiragana, and Katakana. Coders may also search the Dictionary with an English phrase and view Japanese results. Vault supports both full-width and half-width Japanese and English search phrases.
Vault Coder now also offers the ability to display JDrug coding in all Listings and Extracts.
Enablement & Configuration
Coder Administrators must select a JDrug dictionary when assigning one to a Form in Coder Tools.
MedDRAJ
Use Case
Japanese language studies are more holistically supported in CDMS.
Description
With this release, Vault Coder offers MedDRAJ to code and autocode Japanese Adverse Events and Medical History terms.
Vault Coder searches the Dictionary using Kanji, Hiragana, and Katakana scripts to code in Japanese. Coders may also search the Dictionary with an English phrase and view Japanese results. Full-width and half-width Japanese and English search phrases are both supported.
MedDRAJ Synonym Lists, which generate relevant Japanese suggestions from the Synonym List, are also available with this release. All Listings and Extracts are capable of displaying MedDRAJ coding and upversioning both MedDRAJ Forms and Synonym Lists.
Enablement & Configuration
Coder Administrators must select a MedDRAJ dictionary when assigning a dictionary to a Form in Coder Tools > Study Settings > Form Configuration.
Study Design & Configuration
Features in this area apply to Studio, the study design and configuration area for Veeva EDC.
Aggregate Identifiers & Functions
Use Case
This feature increases the variety of rules that can be written by allowing Study Designers to consider values of an identifier as an array. An example would be being able to return the maximum of all dates entered into a repeating item group, or across multiple repeating forms.
Description
Study designers can now specify if an identifier should return an array of values, instead of distinct values. For the repeating parts of an identifier, they can use an asterisk (*) instead of a number inside the brackets to indicate that all instances of repetition should be considered in the value of the identifier.
Aggregate identifiers can’t be used standalone in an expression. They must be used within an aggregate function. Aggregate functions include:
- Sum
- Min
- Max
- First
- Last
- FindValue
These aggregate identifiers cannot be used standalone in an expression and need to be used within an aggregate function (like Sum(), Max(), …)
Enablement & Configuration
This feature is available automatically in the Rule Editor for Studies using version 2 of the expression grammar.
Cross Vault Copy
Use Case
This feature allows users to better leverage template vaults, which provide increased security for library collections.
Description
The Copy From Study dialog now allows study designers to select the vault they want to copy from, as well as the study and environment. Users can copy definitions (Event Groups, Events, Forms, Codelists, Units, and Rules across selected vaults. Previously, users could only copy from studies within the current vault. This allows users to copy definitions from template vaults or even UAT or production vaults when necessary.
Enablement & Configuration
This feature is available automatically for all Studies using automatic deployments.
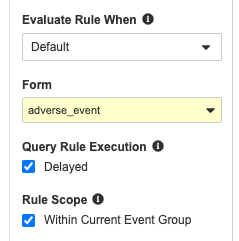
Delayed Execution for Query Rules
Use Case
With recent enhancements to rules functionality, rules may take longer to process after form submission, as they need to gather more data points across the schedule. We recommend using asynchronous rules when the expression contains a Form Link or aggregate identifier, or if the rule would result in a reciprocal evaluation, to help maintain form submission performance.
Description
Study designers can mark query-type Rules as having “delayed execution”, meaning that after a Form is submitted, the rule enters a queue for processing, instead of processing immediately after form submission. As a result, queries created by these asynchronous rules don’t display directly after the post-submission form refresh. Instead, these queries will display anytime after the application is refreshed, for example, when navigating to a different Form.
Some rule types already have this delayed behavior, such as Add Assessment, Create Protocol Deviation, Send Email, and Override Review Plan rules. These rules remain fully asynchronous, without study designers having to mark them as delayed.
In support of this feature, we added an error console to EDC Tools > Rules that displays any errors that occur during the execution of asynchronous rules. Vault keeps errors in the console for sixty (60) days.
Enablement & Configuration
This feature is available for Studies using version 2 of the expression grammar. Study designers must configure this feature for individual rules.
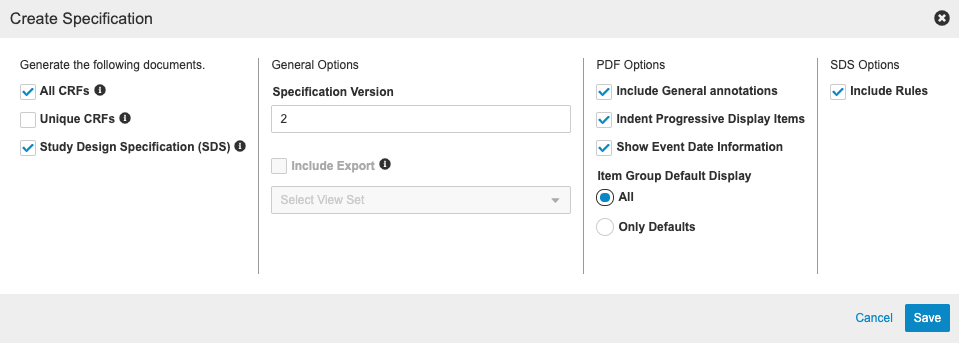
Annotated PDF Enhancements
Use Case
Enhanced usability provides more support for design validation and documentation.
Description
This release includes the following enhancements to annotated PDFs::
- The summary now includes the build number along with the casebook version
- Users can enter a specification version when creating the documents .
- There is better support for the display of default Item Groups.
- Annotations for the Codelist and Unit Names.
- Annotations for disabled rules (if this is not a progressive display study) for studies using rule v1 and studies created prior to 20R1
- Annotations for Item Groups
- Annotations for Event to include Event Date
- Annotations for SDTM at Form and Item
- Formatting changes including text color and the Form Name is now inline with the rest of the Form.
There is now also an updated dialog for annotated PDF creation.
Enablement & Configuration
These changes apply automatically.
Change Vault Login to Vault Selector for Diff Reports
Use Case
This feature allows SSO users to run a comparison report across vaults without having to use a non-SSO account.
Description
With this release, users no longer have to log in to a remote vault to run a comparison report. This process will provide users with the same list of vaults that they have access to at the Vault level and will accommodate SSO users.
Enablement & Configuration
This change applies automatically.
SDS Updates
Use Case
These updates provide additional information for study designers and data management and improve usability of the specification.
Description
The SDS has been updated for general quality improvements including the following:
- Schedule tree updates - like adding names for event group and form
- Schedule Grid updates - like adding the event group
- Carrying labels down through columns like the names
- Removing the system rules from the rules tab and adding rule bindings
- Adding a local lab configuration tab
- Adding additional information to the summary page
Enablement & Configuration
This feature is available automatically.
Access Current Review Plan in a Rule Expression
Use Case
This feature allows more granular control of override review plan rules by allowing study designers to check if a subject is on a specific review plan before deciding if the review plan needs to be overridden.
Description
Study designers can now access the Review Plans currently assigned to a Subject in a rule expression by using two new @Casebook attributes: current_sdv_plan__v and current_dmr_plan__v.
Enablement & Configuration
These attributes are automatically available for Studies using version 2 of the expression grammar.
Study Administration
Features in this section apply to System Tools or EDC Tools, a study-level administration area for Veeva EDC.
Allow Deletion of Sites in Production
Use Case
If a Site is created in the wrong country by mistake, users can delete that Site in Production.
Description
Users can delete a Site in EDC Tools in Production environments if there are no subjects in that Site.
Enablement & Configuration
Auto-on.
Study Data Extract Enhancements
Use Case
This feature allows users to include more options for their exports.
Description
With this release, multiple enhancements have been made to the Study Data Extract job, including the addition of new columns to clinical datasets, supporting custom objects information extraction, and additional configuration options in the SDE dialog UI and FTP connection dialog. The new features are listed below:
- Add Data Type to Key columns and Length column in Definitions File
- Populates the data type (such as text, integer) for all columns in the Definitions file for SAS and CSV exports and adds a Length column to CSV exports
- New checkboxes in the SDE dialog UI for:
- Using External ID instead of Item Definition Names in clinical datasets
- Split clinical Datetime columns into two columns: Date and Time
- Include forms intentionally left blank
- Adds a FORMILB column for intentionally left blank forms in clinical datasets
- Exclude blank forms
- Add a LINKEDTO column for form linking datasets
- Adds a column to show how many forms are linked to that dataset and includes some of the item data within those linked forms
- Users can choose a Zip File Name for SDE export
- Allows users to enter a custom zip file name instead of a system-generated one
- Choose FTP directory path where SDE files will be placed
- Allows users to specify a directory path they want the SDE files to go to - they are allowed up to three directories within the path
- Add subject status dates to SYS_SUB
- The SYS_SUB dataset now contains columns for all subject status change dates. Those dates get set through rules when the subject status gets updated.
- Export Custom Object files in the SDE
- Allows users to add records to a custom object configurator object in Business Admin, and the SDE will export those custom objects into files based upon the records.
- New SYS Datasets:
- If Assessments are enabled:
- SYS_ASM
- SYS_ASMR
- If Protocol Deviations are enabled:
- SYS_PD
- If Randomization is enabled:
- SYS_RAND
- If Local Labs are enabled:
- SYS_LABRANGES
- SYS_LABLOC
- SYS_ANALYTES
- If Assessments are enabled:
In 21R2, the Datetime in the Site’s timezone and the Datetime in the timezone of the user running the job will be treated as text and will no longer use the SAS formats, such as DATETIME22.3.
Enablement & Configuration
These enhancements are available to all users that can run or schedule the Study Data Extract job. Extracting custom object data requires a study-by-study configuration in Business Admin to define which custom objects need to be extracted for which study.
Support Multiple Site Selections in Review Plan Assignment Criteria
Use Case
Users can assign plans to selected Sites individually or as a group.
Description
With this release, we allow lead data managers to apply review plan assignment criteria to Sites or Countries as a group or individually. Lead data managers can choose to assign Review Plans for each Site, by Countries, or by Sites using Dynamic Review Plans. If you apply assignment criteria by Sites or Countries individually, then selected override plans apply to each selected Site or Country. If you apply assignment criteria by Sites or Countries as a group, then selected override plans apply on a first come, first served basis for all selected Sites or Countries.
Enablement & Configuration
A Vault Administrator must enable this feature in a study by setting Review Plan Assignment Version to 3 on the Study Configuration record.
Randomization
Features in this section are new features for the Randomization module of Veeva EDC.
Send Email When Subject is Randomized Rule
Use Case
Defined user groups will be notified when a subject is randomized.
Description
With this release, users can configure an email to be sent when a subject is randomized.
Enablement & Configuration
This feature is available automatically in Studies where Randomization is enabled.
Labs
Features in this section are new features for the Labs module of Veeva EDC.
Override Normals Report
Use Case
This report provides insight into whether Normal Ranges need to be updated to reflect the correct normals.
Description
The Override Normals Report displays records where the Site has entered override values on the Lab Form.
Enablement & Configuration
This feature is automatically enabled in Studies where Labs is enabled.
Diff Report for Analyte Library, Lab Codelists, and Lab Units
Use Case
This allows users to compare vaults and see differences before deployment.
Description
In the vault-level comparison report, we now include differences in the Analyte Library, Lab Codelists, and Lab Units. These show the differences between the two vaults.
Enablement & Configuration
This feature is automatically enabled in Studies where Labs is enabled.
Incomplete Status for Normals
Use Case
This status provides insight to where units need to be updated for Lab Normals.
Description
The new Incomplete status for Lab Normals is applied when a Lab Normal is missing data points, most commonly missing units.
Note: This feature will not be available in pre-release until Friday, July 30.
Enablement & Configuration
This feature is automatically enabled in Studies where Labs is enabled.
Pending Approval Count for Normals
Use Case
This a helpful visual indicator of how many Normals need approval.
Description
The new Pending Approval column displays the number of Normals waiting to be approved.
Enablement & Configuration
This feature is automatically enabled in Studies where Labs is enabled.
Role Management & Security
Features in this section are enhancements to the System Tools > Role Management and System Tools > Users areas, as well as changes to standard Study Roles, security, and access control in Veeva Clinical Data.
Multi-role Security (Limited Release)
Use Case
Users can have multiple roles in a study. A user may be a lead data manager, but that user can also be assigned a CRA role to preview the study as a CRA would view it in the Review tab.
Description
Vault CDMS now offers a security model that allows users to have multiple Study Roles in the same Study. A user can have up to 15 distinct roles in a vault, either across the same study or across multiple studies. This model leverages the Role Based Security feature. If you have more than one Application Role in a study, resulting access to a study is the combination of all your permission across the roles. All users will now share a common security profile, CDMS All Access.
Enablement & Configuration
Contact Veeva Services to discuss enabling this feature in your vault.
LMS Enhancements
Use Case
Users can now create a Site without needing a study design.
Description
With this release, we made the following enhancements in support of the LMS integration:
- If a Casebook Definition does not yet exist for a Study, the Active Version field on Sites is no longer required. This allows users to configure and begin training for a Study before a study design is complete.
- The Get Enrollment check now occurs every 12 hours, instead of every 24 hours. Vault checks for enrollment at 12:00 AM and 12:00 PM, based on the vault’s timezone.
- The LMS Integration feature is now enabled using the Study Settings object, instead of the study’s Study Configuration record. To enable the feature with this release, a Vault Owner must edit the “lms.enabled” record for the Study to set the Value field to “true”. For any Studies where the integration was already enabled, the “lms.enabled” record automatically has the value set to “true”.
Enablement & Configuration
These changes apply automatically.
Study Role Enhancements
Description
With this release, we made several improvements to the available permissions and standard Study Roles.
We added the following new permissions:
- View Users: Controls the ability to view Users from Tools > System Tools > Users
- Assigned to the standard CDMS User Administrator role and any custom Study Roles that have the Manage Users permission assigned
- View Query: Controls the ability to view Queries in EDC
- Assigned to the following standard Study Roles and any custom Study Roles that have the Open Query, Answer Query, Close Query, or Close All Query permissions assigned:
- CDMS Clinical Coder
- CDMS Clinical Coder Administrator
- CDMS Clinical Coder Manager
- CDMS Clinical Research Associate
- CDMS Data Manager
- CDMS Lead Data Manager
- CDMS Principal Investigator
- CDMS Sub Investigator
- CDMS Study Designer
- CDMS Librarian
- Assigned to the following standard Study Roles and any custom Study Roles that have the Open Query, Answer Query, Close Query, or Close All Query permissions assigned:
- View Study Sites: Controls the ability to view Sites in Tools > EDC Tools > Sites
- Assigned to the standard CDMS Lead Data Manager, CDMS Study Designer, CDMS Librarian, and CDMS User Administrator roles and any custom Study Roles that have the Edit Study Sites permission (formerly known as Manage Study Sites) assigned
We granted the View Casebook permission to all Study Roles, both standard and custom, that have the Data Entry permission assigned.
We granted the View Casebook, Schedule Reports, and Reports Dashboards Tab permissions to the CDMS Auditor Read Only study role.
We relabeled the following permissions:
- Manage Study Sites as Edit Study Sites
- Manage Users as Edit Users
- View Query (existing permission for viewing queries in Workbench) as View CDB Query
We created the following new standard Study Roles:
- CDMS Super User: Users with this study role can access all areas of the application and perform all actions within those areas. This role is in the Administration query team. This role is only available in non-production environments.
- CDMS API Read Only: Users with this study role have read-only access to the EDC API. This role is in the Other query team. This role has the following permissions:
- API Access
- View Query
- View Study Sites
- View Casebook
- View Code
- CDMS API Read Write: Users with this study role have read and write access to the EDC API. This role is in the Other query team. This role has the following permissions:
- API Access
- Add Casebook
- View Casebook
- Data Entry
- View Code
- View Study Sites
- Edit Study Sites
- View Query
- Open Query
- Answer Query
- Close Query
Because these Permission Sets are no longer used by standard Security Profiles, we appended “DEPRECATED” the Labels of the following Permission Sets:
- CDMS Definition Objects Read Only
- All Access Actions
- Base CRA Permissions
- Base Data Manager Permissions
- Base EDC User Permissions
- Base Site User Permissions
- Base Standard Template Report Permissions
- Business Administrator Actions
- EDC Clinical Coder
- EDC Clinical Coder Administration
- EDC Investigator Permission
- EDC Reviewer Permissions
- EDC Study Tools Permissions
- External User Actions
- Full User Actions
- Monitor User Actions
- Read-only User Actions
- Site User Actions
- System Administrator Actions
Enablement & Configuration
These changes apply automatically, with no additional configuration required.
Relabeled CDB's "View Query" Permission as "View CDB Query"
Description
With this release, we relabeled the CDB permission, View Query, as View CDB Query, to prevent confusion with the new permission for EDC queries, View Query.
Enablement & Configuration
The label change applies automatically.
Connections & Integrations
Features in this section are new connections or integrations with Veeva Clinical Data or enhancements to existing ones.
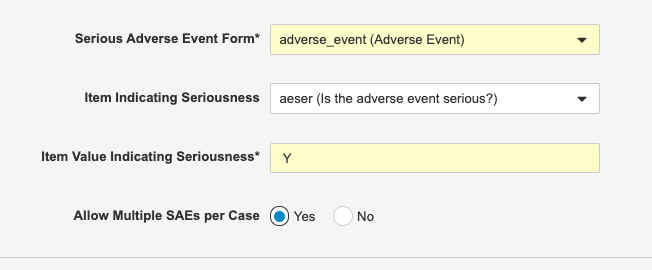
Multiple SAEs per Safety Case
Use Case
Combining SAEs into a single Safety Case reduces the need for manual data entry in the safety system..
Description
Safety Link can now be configured to include multiple Serious Adverse Events (SAEs) in a single Safety Case. This is helpful when capturing a series of related SAEs. For example, if a subject was hospitalized for a heart attack and then, a week later, had a stroke during the same hospital visit, both of those SAEs would be included in the same Safety Case.
Enablement & Configuration
Safety administrators must enable this feature by setting Allow Multiple SAEs per Case to Yes from Tools > EDC Tools > Safety Configuration.
Send Protocol Deviations to Vault CTMS
Use Case
Protocol Deviation records created in CDMS can now flow automatically into CTMS for a centralized location for managing Protocol Deviations.
Description
With this release, Protocol Deviations created in Vault CDMS can be configured to flow into Vault CTMS via the Spark Connection. Once configured, creating or updating a Protocol Deviation in CDMS (either programmatically or manually) triggers an action in CTMS to either update or create the matching Protocol Deviation in CTMS. Protocol deviation data only flows one direction (into CTMS) with the Spark Connection. Any updates made in CTMS don’t transfer back into CDMS.
Enablement & Configuration
This feature requires a Vault CTMS Connection. Categories, Subcategories, and Severities must be configured within CTMS and mapped to the corresponding CDMS records to enable the transfer.
Safety Integration Enhancements
Use Case
These updates offer enhanced integrations with third party safety systems.
Description
Safety Link now offers the ability to indicate that the Investigational Product is blinded and configurable to use a NullFlavor of 99999999 for coding.
Enablement & Configuration
Safety administrators can configure these options in Tools > EDC Tools > Safety Configuration.
Migrations Product Toolkit: Loader (Limited Release)
Use Case
Organizations can now explore feasibility of, and conduct migrations from, supported source EDC systems to Vault CDMS.
Description
The Vault CDMS Migrations toolkit is a set of tools designed to help users migrate data from various platforms into Vault CDMS EDC. This set of solutions assists users in rebuilding existing study elements (Builder), loading source data (Loader), and verifying the process (Validator). With this release, Veeva Services now has the ability to use Loader to perform various semi-automated study migration operations, including EDC subject creation, event creation, form creation, and item value population, along with support for basic transformations during the ingestion process.
Enablement & Configuration
Contact Veeva Services to discuss migrations for your organization and the appropriate transition strategy based upon your needs. In the current release, this feature is only available to Veeva Services, as they will perform any migrations on your behalf.
Clinical DataBase (CDB) & EDC Clinical Reporting
The following are new features for the Veeva CDB application, EDC Clinical Reporting (the Veeva Clinical Data solution for data cleaning and reporting), or both.
Availability: Clinical DataBase (CDB) is only available to CDB license holders. Contact your Veeva Services representative for details.
Import Monitoring & Updated Notifications
Use Case
These changes provide more detailed information about data loads that wasn’t previously available and eliminate redundant information.
Description
With this release, Workbench now only sends an email notification when data reprocessing (after a new package is imported into another source) results in changes to the issue log. Prior to this release, Workbench sent an email notification upon the completion of any reprocessing. This created a lot of noise and made it difficult for recipients to determine if there were any changes that they needed to make.
We also made the following enhancements to the data import process and UI to provide additional information about data loads:
- For each package processed, Workbench creates a log file with details about the import job. The log file contains the start and completion times for the transformation and import states of the job, as well as the duration of transformation and import. Users can download the log file, named “{StudyName}-{SourceName}-{yyyy}-{MM}-{dd}T{HH}-{mm}Z” using the import date and time in UTC, from Import > Packages.
- We updated the name of the Issue Log download file to “{StudyName}-{SourceName}-{yyyy}-{MM}-{dd}T{HH}{mm}{ss}Z.csv” to match up with the log file.
- We added the Deployed column to the package list, which displays the datetime that the source was put into use. This is useful for determining if the data was ever displayed in Workbench. If a new package for the source is imported before the original finishes processing, Workbench switches to the newest package, and so the original package’s data never displays in a listing.
Enablement & Configuration
These changes apply automatically for all imports following the release.
Workbench Labs Data Reformatting
Use Case
The new CDB format for data that comes from EDC’s Labs module is easier to clean, review, and export.
Description
With this release, the Local Labs data coming from EDC Labs Module will be restructured in CDB. Previously, the Local Labs data was displayed in a wide format with separate sets of columns for each analyte and its related fields. For example, if a lab form contained three analytes, three sets of columns would be displayed, one for each analyte. As part of this feature, like columns are consolidated to display the data in a long format. In the same example form with the three analytes, only one set of columns would now display.
If a lab contains a lab panel, Workbench turns the lab panel into a repeating Item Group, in which each Analyte_is an instance of the _Item Group. Workbench displays all possible columns by default and converts some data types into strings. By default, Workbench displays decode, uom, translated, and uom translated columns. If a column is not applicable to the data type of the analyte, Workbench shows a blank cell. For example, if the LBORRES data type is unit, the DECODE_LBSTNRC column is not applicable and shows blank cells. See the table below for the default analyte columns in a lab form and their data types.
| Column Name | Analyte Data Type | Column Data Type |
|---|---|---|
| LBTEST | All | Text |
| LBORRES | All | Text |
| DECODE_LBORRES | Codelist, Unit, Number * If the LBORRES data type is Unit, this column contains the UOM for LBORRES |
Text |
| TRANSLATED_LBORRES | Unit | Text |
| UOMTRANSLATED_LBORRES | Unit | Text |
| LBORNRLO | Unit, Number | Number |
| UOM_LBORNRLO | Unit | Text |
| LBORNRHI | Unit, Number | Number |
| UOM_LBORNRHI | Unit | Text |
| LBOVRDNRLO | Unit, Number | Number |
| UOM_LBOVRDNRLO | Unit | Text |
| LBOVRDNRHI | Unit, Number | Number |
| UOM_LBOVRDNRHI | Unit | Text |
| LBSTNRC | Codelist, Text | Text |
| DECODE_LBSTNRC | Codelist | Text |
| LBOVRDNRC | Codelist, Text | Text |
| DECODE_LBOVRDNRC | Codelist | Text |
| LBNRIND | Codelist, Text | Text |
| LBCLSIG | Codelist, Text | Text |
Additional CQL functions are now available for Local Labs data coming from EDC:
| CQL Function | Description | Valid Items to Use with the Function |
|---|---|---|
SpecimenType() |
Returns the Specimen Type of the Analyte | LBTEST |
TestingMethod() |
Returns the Testing Method of the Analyte | LBTEST |
LOINCCode() |
Returns the LOINC Code of the Analyte | LBTEST |
AnalyteCode() |
Returns the SDTM Code of the Analyte | LBTEST |
LabLocTitle() |
Returns the Lab Location Title | LBLOC |
OverrideReason() |
Returns the Override Reason | LBOVRDNRLO, LBOVRDNRHI |
Enablement & Configuration
After a Workbench Export job, any core listings that contain data from the Labs module are automatically updated to the new format and structure. The CQL for existing listings that references old Labs data from EDC should be checked and, if needed, adjusted.
3rd Party Data Import Enhancements
Use Case
These changes enhance data ingestion to support additional data scenarios.
Description
With this release, the “site” key in the manifest file is optional. When the manifest file doesn’t include a “site” key, CDB expects that all Subject IDs are unique within the Study. If CDB finds a row with a duplicate Subject ID, it skips the row and records the skip in the Issue Log. Users can resolve this by updating their configuration in EDC to prevent duplicate Subject IDs.
Users can now also set a default Event for a given data load. If the data doesn’t include Event information, the manifest file can define a default Event to import the data into. This can occur at the package (source) or file (form) level. Using this event default in the manifest eliminates the need for data vendors to modify the data files to add an Event column and enter into the defaulted value for each row.
Enablement & Configuration
This feature is available automatically. Following the release, users can begin importing packages with manifest files reflecting these changes.
CQL Improvements
Description
On Subject
This feature introduces a new form modifier: ALIGN and UNALIGN. On Subject was a feature introduced in 21R1 that allows the user to specify that they want to join two forms together only on subject, instead of subject and event, which is the default behavior. Prior to this release, when using On Subject with a Form or Item Group that was repeating, CQL only returned joined data for the matching sequences. Now, CQL gives users control to either continue to only return data for joined sequences or return data even when the sequences don’t align. For example, when showing information from a Demographics form and a repeating Adverse Event form, CQL now returns the data from the Demographics form in the rows returned for all Adverse Event form instances for a subject. This behavior is “unaligned”. It is returning the data even when the sequences are not aligned. When using On Subject, the default behavior is UNALIGN. To continue using the previous behavior (always aligning the data on sequence), users must specify the ALIGN attribute with On Subject.
select @HDR.Event.Name, @HDR.Subject.Name, d.GENDER, ae.AETERM, ae.AESEV, ae.AETOXGR, ae.AEENDTC from Demographics d, Adverse_Events ae On Subject Unalign
where (@Form.Status = 'submitted__v' or @HDR.Event.Status IN ('did_not_occur__v'))
Raw and Imputed Dates
CDB supports three different ways of looking at dates and datetimes captured through EDC: imputed, raw, and site normalized. Imputed dates are dates where logic is applied to impute any missing parts (unknowns) in a date or datetime. In EDC, studies can allow the collection of unknown day, month, or hour parts of dates and datetimes. In CDB, when a date contains an unknown, CDB applies first logic to those unknown parts. If the month is unknown, CDB imputes the first month of the year. If the day is unknown, CDB inputs the first hour of the day. By default, all dates and datetimes are returned as whole date and datetimes where the unknown parts are imputed. This is done to make date and datetime comparisons easy for review and cleaning. CDB can also return the exact representation of the date and datetime as entered with the RawDate() function. This function always returns the value exactly as entered as a string, and it doesn’t substitute any of the unknown parts. Additionally, CDB can also return dates or datetimes as a site normalized value using the SiteNormalizedDate() function. This function returns dates and datetimes adjusted to match the timezone of the site for which they were captured.
Prior to the 21R2 release, the imputed date and datetimes (the default representation) incorrectly included site normalization. This has been fixed as part of 21R2.
In support of these changes, we added the following CQL functions:
Unknown
Returns the parts of the date that are unknown if the date contains unknown parts, otherwise returns the string COMPLETE.
UnknownImpute
This function allows a user to specify how they want to represent unknown parts. The function, given an Item, determines if a date contains unknown parts using the raw date value, and then it applies the provided logic for how to handle the unknown parts. The result is a value with the date data type. If the value doesn’t contain any unknown parts, the function returns the default date.
UnknownImpute(item, day impute type, month impute type, time impute type)
UnknownImpute(itemX, 'FIRST DAY', 'FIRST MONTH', 'FIRST HOUR')
UnknownImpute(itemX, 'LAST DAY', 'FIRST MONTH', 'FIRST HOUR')
Day
FIRST DAY: First day of the month.LAST DAY: Last day of the month.MID DAY: 15th of the month.
Month
FIRST MONTH: First month of the year.MID MONTH: June (6)
Time
FIRST HOUR: 00:00 24hrLAST HOUR: 23:59 24hrMID HOUR: 12:00 24hr
Enablement & Configuration
These changes apply automatically.
Access Form Links from Workbench
Use Case
This feature allows for the extraction of form linking data out of CDB using the raw export type. Furthermore, the new form attributes help data managers review and clean form links.
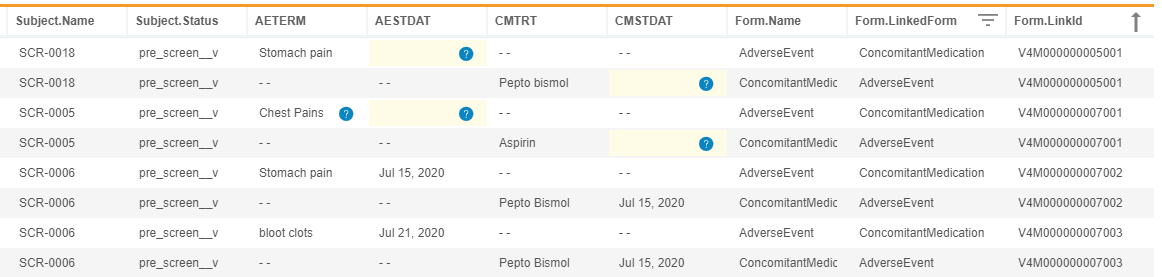
Description
With this release, CDB now imports Form Link data from EDC. Users can return the list of linked forms in a given study by entering CALL Sys_Links into the CQL Editor. The resulting dataset contains the following headers:
Study.NameSite.CountrySite.NumberSubject.NameEventGroup.NameEventGroup.SeqNbrEvent.NameForm.NameForm.SeqNbrForm.LinkCreatedDateForm.LinkID
Each row contains one side of a link. For example, if an AE form and a CM form are linked, two records display, one for each form with the same Form.LinkID. By default, the results are sorted by LinkID. The resulting CQL aren’t further filterable or sortable.
As part of this feature, we introduced new form attributes: @Form.LinkedForm and @Form.LinkId. These attributes help data managers to review and verify form links by allowing them to return columns from linked forms without using a subquery.
For example, the following CQL will return Header information, AETERM and AESTDAT from AdverseEvent form, CMTRT and CMSTDAT from ConcomitantMedication form, Form name, Linked Form Name, and LinkId from Adverse Events and Concomitant Medication forms that are linked together.
SELECT @HDR, AdverseEvent.AETERM, AdverseEvent.AESTDAT, ConcomitantMedication.CMTRT, ConcomitantMedication.CMSTDAT, @Form.Name, @Form.LinkedForm, @Form.LinkId
FROM AdverseEvent, ConcomitantMedication
WHERE @Form.LinkedForm IN ('AdverseEvent', 'ConcomitantMedication')
ORDER BY `@Form`.`LinkId` ASC
The CALL Sys_Links dataset is automatically generated and included in the raw export. The Sys_Links listing behaves the same as the other system datasets in a raw export.
Enablement & Configuration
The changes to import and CQL apply automatically, but Workbench does not add the Sys_Links listing to existing raw Export Definitions. To export Sys_Links, create a new raw Export Definition after the release.
Improvements to System Listings
Use Case
This feature eliminates the need for system datasets, such as Sys_ILB, in the raw export definition to be split into multiple files.
Description
Raw-type Export Definitions automatically contain the following auto-generated System Listings: Sys_Sites, Sys_Subjects, Sys_Events, Sys_Forms, and Sys_ILB. With this release, we’ve updated the CQL of these system datasets as follows:
| System Listing | CQL Syntax |
|---|---|
| Sys_Sites | CALL Sys_Sites |
| Sys_Subjects | CALL Sys_Subjects |
| Sys_Events | CALL Sys_Events |
| Sys_Forms | CALL Sys_Forms |
| Sys_ILB | CALL Sys_ILB |
The headers in these listings are unchanged. By default, all listings, except for Sys_Sites, are sorted by Subject.Name. By default, Sys_Sites is sorted by Site.Name. The resulting CQL isn’t further filterable or sortable.
Enablement & Configuration
These changes only apply to raw Export Definitions created after this release. To apply these changes to existing definitions, a user must edit that definition and save their changes.