What's New in 23R1
Pre-Release Date: March 27, 2023 | CDB Pre-Release Date: April 3, 2023 | Release Date: April 14 & 21, 2023We are pleased to bring you Veeva Clinical Data in 23R1. Read about the new features below. You can find information on enabling new features in the 23R1 Feature Enablement Details. Information on developer features (REST API) is in the Developer Portal.
Clinical Data
Features in this section are changes that apply to all application areas of Veeva EDC and CDB.
Removing Legacy EDC Features
Description
Veeva has removed the following legacy EDC features, which are no longer being used by customers:
- PDF V1
Enablement & Configuration
This change applies automatically.
Data Entry
Features in this section are changes to the Data Entry tab, a working area for investigators and clinical research coordinators to enter study execution data.
ILB No Longer Allowed for Lab Forms
Use Case
This feature removes redundant form actions.
Description
Users can no longer mark a Lab Form as Intentionally Left Blank. Instead, they can mark blank Lab forms as Lab Tests Not Performed.
Enablement & Configuration
This change applies automatically.
Query Detail Support for Multi-role Security
Use Case
This feature creates consistency among single-role security studies.
Description
Users can select the Query Team they want to use when creating a manual query if the user has multiple roles on multiple query teams. The selected Query Team will then be visible in the UI and will display in the Query Detail Listing and the SYS_Q dataset of the Study Data Extract.
Enablement & Configuration
This feature is available automatically in vaults using Multi-Role Security.
Study Closeout: Non-restricted Version
Use Case
Users no longer need to create custom roles to download and accept non-restricted Closeout PDFs.
Description
With this feature, Vault will check if a site contains any restricted forms when generating Closeout PDFs. The Closeout PDF won’t be marked as restricted if no restricted data is detected. Site users won’t be required to have the restricted data permission in order to download and accept the files.
Enablement & Configuration
This feature is available automatically.
UI Text Changes for Form Submission
Use Case
This feature ensures that site users are submitting form data.
Description
With this feature, the “Complete” button on forms has been relabeled as “Submit.” Users will see a warning dialog if they navigate away from an In Progress form without first submitting the data to ensure that data is submitted. When prompted with the warning dialog, users can choose to submit the form, continue editing the form, or leave the page.
Enablement & Configuration
These changes apply automatically.
Clinical Coding
The following are new features for Veeva Coder, the clinical coding area for Veeva Coder.
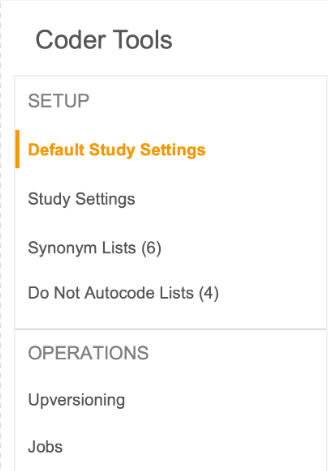
Coder Tools Navigation Refresh
Use Case
This new UI experience is scalable as we add more features to Coder Tools.
Description
With this feature, the main navigation in Coder Tools will be moved to the left panel.
Enablement & Configuration
Auto-on.
Sync Synonym Lists
Use Case
Autocoding and Suggestions will be consistent across all Studies that span two Vaults.
Description
Users can designate one Synonym List and Do Not Autocode List to perform all autocoding and suggestions actions for two vaults. Autocoding and Suggestions will be consistent across all Studies that span two vaults.
Coders can’t Propagate Code and Coder Managers can’t apply their approval action to the Synonym List on the secondary vault. While all updates to the Synonym List will sync across two vaults, the action to propagate the code to all Forms that are assigned to the same Synonym List is limited to the primary vault.
Enablement & Configuration
Contact Veeva to enable.
UI for Orphaned or Deleted Coder Items
Use Case
Study Designers will have options to fix the Coding Configuration instead of requiring assistance from Veeva Support.
Description
With this release, Study Designers can still see coding configurations for Form and Item Definitions from an orphaned Verbatim Item Definition or an orphaned or deleted related coding Item Definition. Study Designers can then select a new Item Definition or make the mapping not required (in the case of related Coding Item Definitions only). Users can also delete the Coding Configuration on the Form Definition entirely.
Enablement & Configuration
Auto-on.
Assessments
The following are new features for the Assessments area of Veeva EDC.
Medical Assessments Enhancements
Use Case
Medical Assessors can now directly view assessments and assessment questions in the audit trail within the assessment module and PDF.
Description
This release includes the following enhancements to Medical Assessments:
- Added the Medical Assessments audit trail to the UI and PDFs
- The audit trails for assessments and assessment questions are now accessible from the Medical Assessment UI and can be included in the assessment PDF along with supplemental data audit trails
Enablement & Configuration
These changes apply automatically.
Study Design & Configuration
Features in this area apply to Studio, the study design and configuration area for Veeva EDC.
Remove Subject Status Date on Status Rollback
Use Case
This change improves support for CTMS-integrated Studies.
Description
As a subject progresses through a Study, Vault updates its Subject Status. When a subject’s status changes, Vault records the date that a subject entered that status. With this release, when data changes for a subject, if a Set Subject Status Rule that previously evaluated as true is now false, Vault clears the associated status change date.
This feature adds a new setting to Studio > Settings, Enable Subject Status Date Rollback.
Enablement & Configuration
For existing Studies (created before 23R1), a study designer must enable this feature from Studio > Settings. For new Studies created after 23R1, this feature is automatically enabled.
Learn More
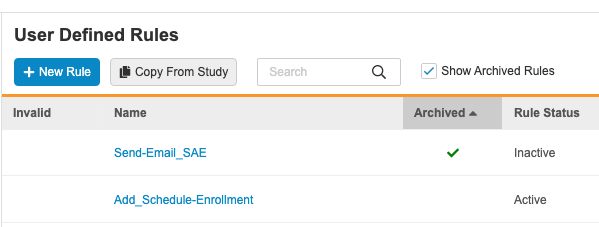
Rules Archive
Use Case
This simplifies the deployment of rules. Users are now also able to restore deleted rules and view deleted rules for reference purposes.
Description
When deleting rules, Vault now marks those deleted rules as Archived. After a user executes the Delete action against a Rule, Vault applies the Archived status, marks the rule as Inactive, and hides the rule from the User Defined Rules listing. Studio users can select the Show Archived Rules checkbox to show archived rules in the list. Archived rules have a green checkmark () in the Archived column.
Users can then view and restore these archived rules. Archived rules behave in the same way as Inactive rules. Vault excludes these rules from validations and documentation, deploys them (in the Archived status), and prevents users from marking them as Active. That prevents these rules from executing.
Enablement & Configuration
This feature is automatically enabled. It applies to all rules versions.
Learn More
SDS Enhancements
Use Case
This feature provides better usability of the SDS.
Description
This release includes the following SDS enhancements:
- Added columns for missing properties and system edit checks to the Schedule Tree tab and updated column headers and order of columns for consistency
- Added missing columns to the Form Definitions tab, relabeled column headers to match UI, and removed obsolete/retired columns
- Added Last Modified columns to Form Definitions and Schedule Tree
- The “Include Rules” option is now checked by default when generating an SDS from Studio
- Updated formatting of the Review Plan tab including newly-formatted rows for different object types to match the Form Definition tab and carried down column values for filtering
- Removed obsolete columns and added a Form Label column in the Rules tab
- Added Form Name and column headers to Schedule Grid tab
- Updated the Summary tab for clarity
- Added and updated columns on the Codelists and Unit Codelists tabs
- Added Object Name columns to the Date Comparisons tab
Enablement & Configuration
These changes apply automatically.
Sort @PreviousEvent Rules by Schedule
Use Case
When locating the Previous instance of an Event or Form, Study Designers do not always want to sort by Event Date, and instead sort based on the order of Events defined in the Studio Schedule. This feature adds additional flexibility to the new @PreviousEvent rule functionality added in the last release.
Description
Rules containing a @PreviousEvent identifier can now be configured to sort by Schedule order.
Enablement & Configuration
This feature is automatically available for use in Studies using Expression Engine V2.
Learn More
Studio Usability Improvements
Use Case
These changes improve the usability of Studio to speed study build delivery.
Description
This release includes several usability improvements for Studio:
- Schedule is now the default landing page when opening Studio.
- The Comparison Report (diff report) no longer reflects changes to the rule action order that are controlled by the system.
- We made some minor formatting, processing, and logging changes to export and import for study languages.
- We relabeled the Future Date property as No Future Date to clarify the purpose of this property.
- The default value for Repeat Maximum is now “50” instead of “9999”.
- Studio users can edit library settings and some study settings after a Study is deployed.
- If no Form Links are included in the study’s schedule, Studio doesn’t run validations against Form Links.
- We added validation errors for unsupported uses of cross-form derivations.
- Cross-form derivation rules that cause a circular reference between items will trigger a warning instead of a casebook validation error when the circular reference exists only within a single form.
Enablement & Configuration
These changes apply automatically.
Study Administration
Features in this section apply to System Tools or EDC Tools, a study-level administration area for Veeva EDC.
Disable Manual Casebook Creation
Use Case
Customer’s with IRT (Interactive Response Technology)-enabled studies need a way to block casebook creation through the EDC UI. For these studies, new casebooks should only be created from external integration. This feature removes the need to create custom roles without the Add Casebook permission.
Description
EDC Tools now provides a setting for IRT-enabled studies that disables the + New Casebook button in the Data Entry tab. When the Disable Manual Casebook Creation checkbox is selected for a study, new casebooks can only be created via the external IRT integration. Users must have the Edit Study Settings permission to modify this setting.
Enablement & Configuration
This feature must be enabled in a Study via the Disable Manual Casebook Creation checkbox in Tools > EDC Tools > Settings.
Audit Event for Item Change Reason on Create
Use Case
This feature supports migrations.
Description
With this release, Vault will audit an Item’s Change Reason when the Item is created to support migrated data. If a migrated item has a change reason, the reason will be available in the item’s Audit Trail after the item is created.
Enablement & Configuration
This change applies automatically and is only applicable to migrated studies.
Changes to Planned DateTime & Event DateTime No Longer Included in Audit Trail
Use Case
Parity with migrated data and removal of unnecessary data from the audit trail.
Description
The Audit Trail will no longer display changes to the Event Datetime and Planned Datetime as these fields are no longer used in EDC.
Enablement & Configuration
These changes apply automatically.
Removed Actions Menu from LMS External Connections
Use Case
In the past, there has been a Actions menu icon (gear) denoting an Actions menu next to the LMS connection record that did not function. This icon has been eliminated to remove confusion.
Description
With this release, we removed the extraneous Actions menu icon from System Tools > External Connections > LMS External Connections.
Enablement & Configuration
This feature is available by default.
Review Plan Assignment UI Enhancements
Use Case
These enhancements provide improved navigation and usability of the Review Plan Assignment section, and allow for more granular permission access to the Review Plan Assignment criteria and manual access pages.
Description
With this release we have introduced two new tabs to EDC Tools, Review Plan Assignment Criteria and Review Plan Manual Assignment, that take the place of the single Review Plan Assignment tab and subtabs. In addition, users can now search for site number in addition to site name in a Review Plan Assignment Sites field.
Enablement & Configuration
These changes apply automatically to Studies using V2 or V3 of Review Plan Assignment.
SDE: Add Object Definition Names to Extract
Use Case
This feature will allow users to look up the definition name from the labels to help with downstream analysis and mapping.
Description
In the 23R1 version of the SDE, various Clinical and System datasets will include the following object definition names as new columns: Event Group Definition Name, Event Definition Name, Form Definition Name, Item Group Definition Name, and Item Definition Name.
The datasets impacted are listed below:
- Clinical Datasets
- SYS_EVT
- SYS_FORM
- SYS_ILB
- SYS_LINKS
- SYS_Q
- SYS_PD
- SYS_ASM
Enablement & Configuration
Automatically enabled with SDE version 23R1.
SDE Enhancements
Use Case
These enhancements add more detail to the extract to help users with post-processing analysis of their clinical and operational data.
Description
The following enhancements have been made to the 23R1 version of the SDE:
- Users will have the option to select a boolean formatting type which will determine how booleans are displayed in both clinical and system datasets. This formatting doesn’t apply to the definitions files
- If there are duplicate column headers, both the CSV and SAS files will follow a deduplication pattern of appending _2, _3, etc. to each additional repeating column. When running the “SAS with CSV and XPT”, Export File Type option, users will see an additional column for “SAS Column” in the Study Definitions file so that they can compare the column headers between CSV and SAS for any differences (such as SAS column header truncation for column headers greater than 32 characters in length)
- The rows in the casebook_schedule.csv and casebook_definition_summary.csv files in the definitions folder will be ordered according to the studio study Schedule Design within each casebook version
- The SYS_Q dataset will have a new column for the LASTCLOSEDDT (Query Last Close Date)
Enablement & Configuration
Auto-on with the 23R1 version of SDE
SDE: New Lab Form Format
Use Case
This format makes Lab forms more readable for reviewing clinical data.
Description
In the 23R1 version of the SDE, clinical forms configured with Local Labs will be formatted to decrease the number of columns outputted so that the dynamically-generated Labs columns will be shared across all Lab Analytes on the form instead of having multiple columns per analyte.
Enablement & Configuration
Auto-on with the 23R1 version of the SDE
Enablement Change: SDE SAS Compression
Use Case
SAS zip files will be compressed automatically when running the SDE.
Description
This feature turns on the Enable SAS Artifact Compression feature for all Vaults, which compresses the SAS zip file. SDE Versions 22R1 and later will have SAS compression automatically enabled. Previous SDE versions will be affected by this enablement.
Enablement & Configuration
Automatically enabled in 23R1 for all Vaults.
Study Progress Listing Enhancements
Use Case
With this feature, Vault cleans up additional jobs after the study priority expires.
Description
Users can schedule up to one daily listing job to send to reports, unless the study is set as a priority study. Users can schedule two additional daily jobs for priority studies. When the study’s priority expires, Vault will also automatically expire the additional jobs.
Enablement & Configuration
These changes apply automatically in Studies using the Study Progress Listings in Reports feature.
Study Progress Listing Enhancements
Use Case
These enhancements provide users with more reporting metrics.
Description
With this release, we’ve made some small enhancements to the Study Progress Listings. These changes will be available in the CSV version of the listings that is generated in the Review tab or EDC Tools as well as the Report tab version of the listings.
Query Detail Listing changes:
- New column for Form Status
- New column for number of query messages
Event Progress Listing changes:
- New columns for Event Date frozen and locked
Subject Progress Listing changes:
- New columns for SDV Completion Date and DMR Completion Date
Form Progress Listing changes:
- New columns for Intentionally Left Blank and ILB Date
Form Progress, Event Progress, and Study Summary Metrics Report:
- Values are rounded to the nearest integer
Enablement & Configuration
These changes apply automatically.
Labs
Features in this section are new features for the Labs module of Veeva EDC.
Labs: Support for Multiple Languages
Use Case
Local labs can support multiple languages.
Description
Users can upload translated values for codelists, units, and analytes in Local Labs.
Enablement & Configuration
This feature is available automatically.
Lab Modifier Support for Number Data Type
Use Case
Vault now supports Lab results with “>” or “<” for Number data types.
Description
With this release, users can enable Lab Modifiers for Number data types in the analyte library.
Enablement & Configuration
This feature is available for Studies that have Data Model 2 and Global Labs enabled.
Role Management & Security
Features in this section are enhancements to the System Tools > Role Management and System Tools > Users areas, as well as changes to standard Study Roles, security, and access control in Veeva Clinical Data.
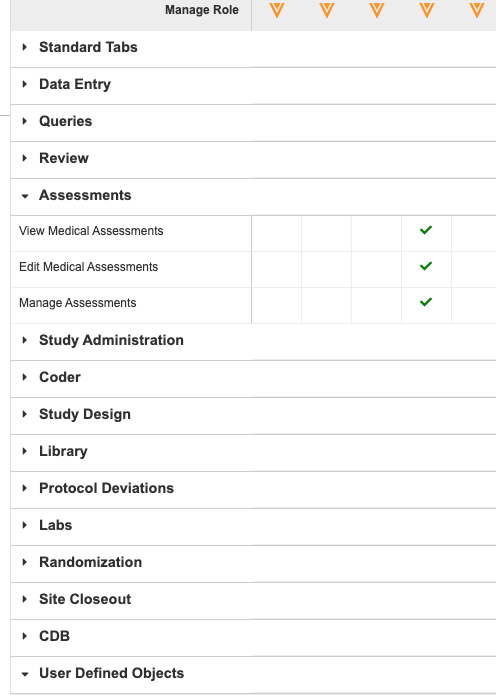
Permission Grouping in Role Management
Use Case
The Role Management grid contains many permissions and has been hard to navigate and find relevant permissions without an inordinate amount of vertical scrolling. This UI enhancement will greatly improve the UX of the Role Management grid, reducing frustration and time spent navigating.
Description
With this release, we have introduced grouping to the Role Management grid in System Tools. Users can expand and collapse these group sections to more easily navigate and find the correct permissions.
We replaced the Permissions section with multiple, categorical sections:
- Data Entry
- Queries
- Review
- Assessments
- Study Administration
- Coder
- Study Design
- Library
- Protocol Deviations
- Labs
- Randomization
- Site Closeout
- CDB
Enablement & Configuration
These changes apply automatically.
User Access Report
Use Case
The User Activity Report was not designed to provide a comprehensive log of all access control related audit data. The User Access Report consolidates all of this historical data, from the creation of the vault, into any easily accessible validated report that can be provided in the event of an audit.
Description
The new User Access Report provides User Administrators with a convenient report that details all access related audit data for users in that vault. The existing User Activity Report historically provided some of this data, but with this release we have introduced a comprehensive chronological log of role assignments and removals, site and country access changes, study assignment and access, and training status changes. The User Activity Report will still be available, but focuses on user activity such as login attempts and password changes.
Enablement & Configuration
This feature is available by default to users with the View Users permission.
User Activity Report Changes
Description
We removed information related to user access (the Current Study Role, Study, Item, Old Value, and New Value columns) from the User Activity Report in preparation for a feature in a later release.
We also added the following tracked events for this report:
- User Unlocked: The user successfully reset their password after being locked out.
- Password Change Unsuccessful: The user attempted to change their password but was unsuccessful.
- Logout Successful: The user logged out of Vault successfully.
- User Inactivated: The user attempted to log in to an inactive account.
Enablement & Configuration
These changes apply automatically.
Study Role Enhancements
Use Case
These enhancements provide permissions to new sections of the application and ensure that roles are able to access and interact with the proper functionality.
Description
With this release, we made the following changes to permissions and standard Study Roles:
- Added the CDB Tools permission and assigned it to the CDMS Data Manager and CDMS Lead Data Manager roles
- Added the Configure CDB permission and assigned it to the CDMS Super User role
- Added the Manage Review Plan Assignment Criteria and Manage Review Plan Manual Assignment permissions
- Assigned the Manage Jobs permission to the CDMS Clinical Research Associate and CMDS Data Manager roles
- Assigned the View Users permission to the CDMS API Read Only and CDMS API Read Write roles
- Assigned the Edit Users permission to the CDMS API Read Write role
Enablement & Configuration
These changes apply automatically.
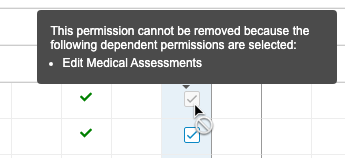
Display Permission Dependencies in Role Management UI
Use Case
Prior to this release, when creating or editing a custom role, users couldn’t easily determine why some permissions were automatically selected, or could not be cleared. This feature provides information on why a certain permission or permissions were added and can’t be removed.
Description
This feature provides a tooltip in the editable Role Management grid when permissions are dependent on an already selected permission.
Enablement & Configuration
This feature is available by default.
Updates to Inactive Users
Use Case
Automatically removing a user’s study access when they have been inactivated will cut down on extraneous clicks, and will ensure that a User Admin does not inadvertently provide study access upon subsequent activation of a user. In addition, previously a user admin would receive a vague error message when attempting to edit an inactivated user’s details. This feature now removes that ambiguity and simply disables the ability to edit the profile of an inactive user.
Description
With this release, the system will automatically remove a user’s study access when they have been inactivated, and will disable the ability to edit an inactivated user’s details.
Enablement & Configuration
These changes apply automatically.
User Import Enhancements
Use Case
The system has always allowed changes to a user’s activation date in individual user management as long as the activation date is not in the past. This feature extends the same behavior to the bulk user import from file feature.
Description
This feature allows user administrators to make changes to a user’s Activation Date creating users via import from file. In addition, if an Activation Date is not specified, Vault uses the current date and indicates that in a warning during import preview.
Enablement & Configuration
These changes apply automatically.
Deployments
Features in this section are enhancements to deployment functionality in Veeva Clinical Data.
Deploy from Development to Validation Environments
Use Case
Previously, the only deployment option to Validation was from UAT. With this feature, deployment administrators can save time by going directly from DEV to UAT.
Description
With this release we have provided the option to deploy from Development environments directly to Validation.
Enablement & Configuration
This feature is automatically enabled.
Prevent Post-Deployment Removal of Roles from Deployment List
Use Case
Previously, in vaults using the Multi-Role Security model, deployment administrators could inadvertently remove a custom role from a target vault if a previously deployed role was removed from the whitelist before a subsequent deployment. This feature removes this possibility by ensuring that a custom role cannot be removed from the whitelist if it has already been deployed.
Description
This feature prevents the unintended removal of custom roles during a vault deployment by disabling the ability to remove a custom role from the deployment list if it has already been deployed.
Enablement & Configuration
This feature is enabled by default for all vaults using the Multi-Role Security model.
Reconcile Missing References During Study Data Copy
Use Case
Previously, if relevant data were actively deleted or created in Production while a Copy Study Data from Production was in progress, the copy job would fail. This feature allows the copy operation to complete even if data was actively being deleted or created.
Description
This feature accommodates active changes to data while a Copy Study Data from Production operation is in process by only copying data that was static at the start of the copy job.
Enablement & Configuration
This feature is available by default.
Connections & Integrations
Features in this section are new connections or integrations with Veeva Clinical Data or enhancements to existing ones.
E2BLink: Duration Inclusion Criteria for Related Forms
Use Case
Previously the only way to include related data to a safety case was through site linking, or very specific (and often less desirable) form designs. The feature allows for more flexible study design (forms), and ability to configure which related information should belong in the case. Some examples of auto inclusion:
- All ongoing and concomitant medications at the time of SAE Start
- All medical history ongoing or ended within 6 months
- Include not just the study drug dispense closest to SAE start (on/before, the pre 23R1 behavior), but also others within 4 days of the SAE Start, on, or after.
- Any other adverse event (serious or not) that has a start date +/- 3 days of the SAE’s start date.
Description
E2BLink now provides the ability to include related information into a safety case beyond just what a site links to the serious adverse event. This includes medical history, concomitant medications, external lab forms, other adverse events, and study drug/product dispenses. Additionally, the ability for forms being linked by the site user as a method of inclusion can be blocked, if desired.
Enablement & Configuration
These settings are automatically available, but a safety administrator must configure these options before they apply to a study.
E2BLink: SAE Not Submitted Email Alerts
Use Case
Safety cases are most urgently time sensitive on the first report from EDC, often just a few days (if not less) for further notification to regulatory agencies. This feature will ensure the timely alerting of cases where sites are omitting important steps that are necessary to ensure the start of safety case data transfer.
Description
This feature accommodates two, important, time-sensitive scenarios:
- The site user has filled out a serious AE form, but has neglected to do the first submit/complete action on the form. That action is what normally begins the safety workflow.
- The site user has filled out a serious AE form, but neglected the AE term, by mistake. The value is required in most safety systems.
Either of these scenarios results in an email alert at 1 hour after the creation of the form. The alert goes to the user who created the form, plus any configured recipients (email groups, distribution lists). The recipients can be configured by country as well, i.e. subjects of specific countries only sent to certain email lists.
Enablement & Configuration
These settings are automatically available, but a safety administrator must configure these options before they apply to a study.
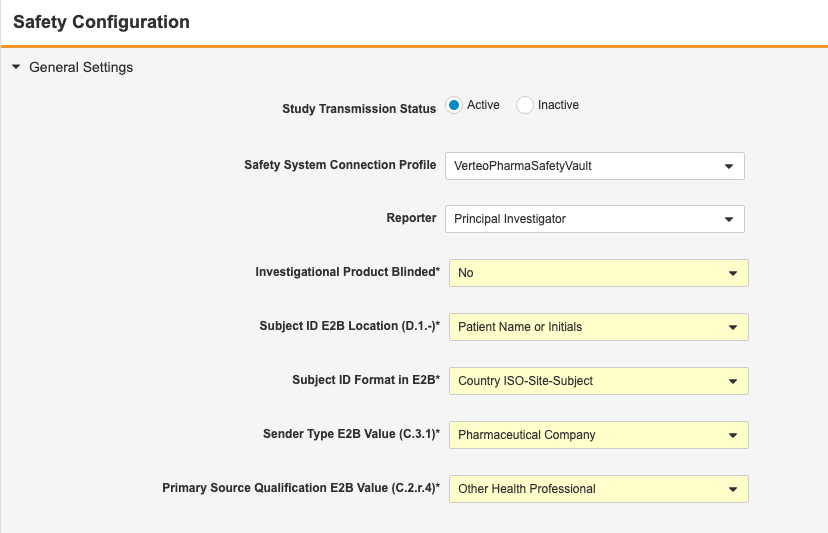
E2BLink: Configure Static Values in E2B XML
Use Case
Safety administrators have further control over what information is included in the safety case.
Description
Prior to this release, E2BLink transferred several static values to the safety system. With this release, safety administrators can configure which values are sent, instead of the CDMS default values for these fields.
The following values are now configurable:
| Field | E2B ID | Default Value | Description |
|---|---|---|---|
| Subject ID E2B Location | D.1 | Patient Name or Initials | Location of the EDC Subject ID in the E2B XML files |
| Subject ID Format in E2B | N/A | Country ISO-Site-Subject | Format of the EDC Subject ID in the E2B XML files |
| Sender Type E2B Value | C.3.1 | Pharmaceutical Company | Qualification type of the assessment of the drug, as it relates to adverse events for the case |
| Primary Source Qualification E2B Value | C.2.r.4 | Other Health Professional | Specialty classification of study drugs, when a site user has indicated that the concomitant medication (in the case data) is classified as Suspect |
| Study Drug Classify when ConMed Suspect | G.k.1 | Always Interacting | For cases when a user is classifying a Concomitant Medication via an Item to Form link, and user chooses SUSPECT, then this classification is to be instead used for Study Drug classifications of the Safety Case. |
Enablement & Configuration
These settings are automatically available, but a safety administrator must configure these options before they apply to a study.
Learn More
Clinical DataBase (CDB) & EDC Clinical Reporting
The following are new features for the Veeva CDB application, EDC Clinical Reporting (the Veeva Clinical Data solution for data cleaning and reporting), or both.
Availability: Clinical DataBase (CDB) is only available to CDB license holders. Contact your Veeva Services representative for details.
Clean Patient Tracker
Use Case
Data managers need to provide complete and clean patient data throughout the lifetime of a study. Today study teams use Excel and/or custom solutions to pull data together then manually mark subjects that are deemed clean and ready for lock. This process is time consuming, limited and prone to errors. With the clean patient grid, data managers will be able to track the cleanliness of the subjects and take direct action on data management cleaning activities.
Description
Data Workbench will provide a new Subject-centric page for data managers to review the cleanliness of each patient. Data managers will be able to filter the grid by cleaning activities and study meta data such as site, country, cohort etc. Data managers will be able to quickly take action on a subject such as reviewing and closing answered queries.
Enablement & Configuration
The Clean Patient Tracker is automatically available.
Clone Listings from Study
Use Case
Clinical trials, especially those associated with the same therapeutic area share similar study designs and have very similar data management plans and reporting structures. When designing studies… reviews, edit checks and reports must be designed and built for each study. This can be time consuming and lead to unnecessary inconsistencies between similar studies. Users will now be able to easily reuse these building blocks, minimizing development time and errors that could be introduced in the process.
Description
Data managers and clinical programmers will also be able to select multiple Listings (Checks and Views) from a defined source study(s) and clone them into their current study. The system will provide a validation summary for each object indicating whether that object is compatible or incompatible with the target study design. The user will be able to review the validation summary, make necessary changes and import the desired cloned objects with their associated statuses (compatible, incompatible). Once imported these cloned objects can be reviewed, modified, and deployed to production.
Enablement & Configuration
The ability to clone Listings from a Study will be auto enabled in the Test environment only. All studies in the test environment, by default, will be set to not cloneable. An administrator will be able to designate which studies can be source study.
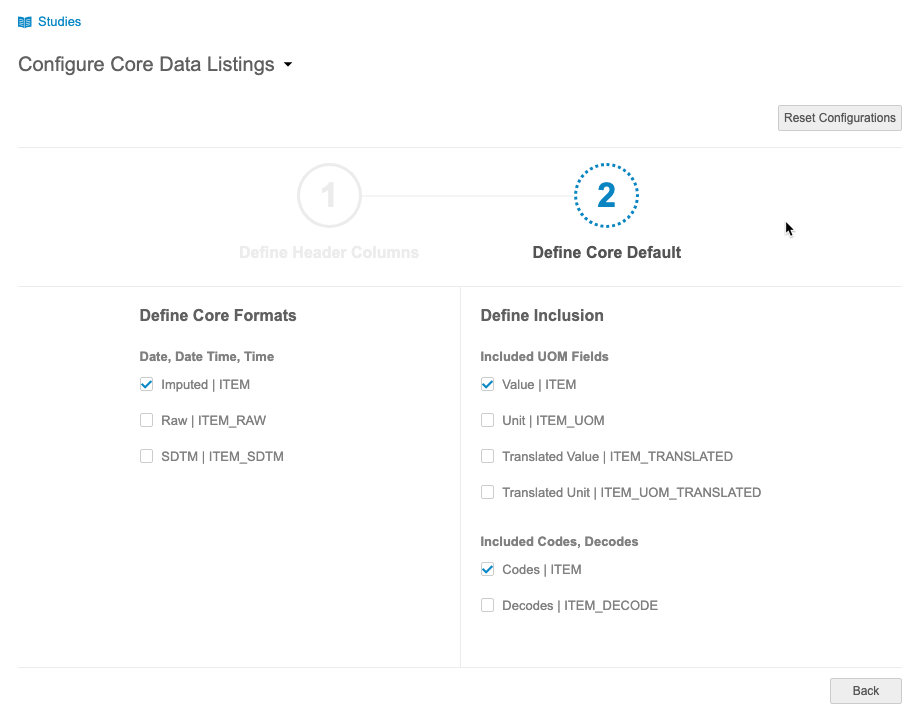
Configure Core Data Listings
Use Case
Core data listings are out-of-the-box listings that correspond to each Form represented in the study. Data managers may prefer a specific format and specific set of study attributes be included in all of Core Listings. With the ability to configure these sets at a vault level, clinical programmers will save a substantial amount of time by not having to recreate each core listing individually.
Description
Administrators can now configure Core Listings. This configuration includes the ability to select which study attributes to include and which formats to use, such as always using raw format for datetimes or always including Translated Value and Translated UOM. Configuration is subject to a review and approval process.
Configurations apply to all Core Listings within a vault.
This feature adds a new Configure Core Data Listings page to CDB.
Enablement & Configuration
The ability to configure core data listings is automatically enabled. For existing listings, the default configuration remains the same. An administrator must review and approve configuration changes before CDB applies the changes.
Third Party External ID Support
Use Case
This provides a method to pass through an identifier for users to identify a row of data that is tied to a query.
Description
Users can provide a row external id in their manifest file when importing non-EDC data to be used to identify a unique row of data that queries are associated with. Once ingested, this Row External ID will be accessible via the @QRY dataset and in the Core Query Listings.
Enablement & Configuration
This feature is automatically enabled. A user must edit a manifest file to use it.
Listing Search & Favorites
Use Case
Navigation is an essential part of the user experience and enables data managers to access data as quickly and intuitively as possible.
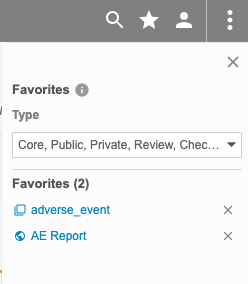
Description
With this release, CDB users can mark listings, checks, and views as Favorites. They can manage their favorites from the Favorites panel, which is accessible via the Favorites () button in the top navigation menu.
|
|
Users can now also search for listings from any page within the application. Search has moved into the top navigation bar. Prior to this release, users could only search from the Listings page.
Enablement & Configuration
This feature is automatically enabled.
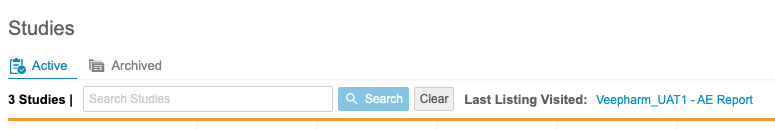
Study Search & Last Listing Visited
Use Case
Navigation is an essential part of the user experience and enables data managers to access data as quickly and intuitively as possible.
Description
Users can now search for specific Studies from the Studies page in Workbench. Workbench also displays a link to the Last Listing Visited on this page, which opens the last listing that the current user visited.
Enablement & Configuration
This feature is automatically enabled.
Fill CQL Function
Use Case
The fill function provides a way to replace the CQL NULL values in a custom listing.
Description
When clinical data items from a non-repeating Item Group and a repeating Item Group are combined in a listing, CQL shows CQL NULL values for non-repeating Items where the Item Group Sequence Number (@ItemGroup.SeqNbr) is greater than 1. Because non-repeating items only have a sequence number of 1, and it isn’t possible to collect data for sequence numbers greater than 1 for non-repeating items. CQL uses CQL NULL to represent item values for which it was not possible to collect clinical data.
The fill function takes one argument which is a data item in a non-repeating Item Group. The fill function will return a new column in which CQL NULL values will be replaced with the argument data item. This function is applied at the form instance level.
Enablement & Configuration
This function is available automatically.
Open Listing in a New Tab
Use Case
Users may need to access multiple listings at one time. With this feature, it’s easier for users to compare different sources of data quickly and efficiently.
Description
With this release, CDB allows users to access multiple listings simultaneously across different browser tabs. Users can open a listing in a new tab.
Enablement & Configuration
This feature is automatically enabled.
Export Audit Logs Track Blinding Changes
Description
CDB now tracks the following changes to package blinding in the audit log for Export Definitions:
- Export definition marked as Blinded
- Export definition marked as Unblinded
Enablement & Configuration
This feature is available automatically. Any changes to package blinding are captured by the audit log following this release.
EDC Migrator
Features in this section are new features for Veeva EDC Migrator.
Create Repaired Forms as Facades
Use Case
When Forms are placed “In Progress” there is the implication to Users that they must submit the Form. With this enhancement, Users can leave the Forms as Facades and not have to submit them. Furthermore, when a Form is first created in EDC, it’s created as a Facade, so this behavior would mimic existing EDC functionality.
Description
This update to the Repair process ensures that all Forms created by repair are created as Facades (blank forms). Prior to this release, these forms were in the In Progress status.
Enablement & Configuration
This is automatically enabled and can’t be disabled.
Migration: UI Redesign - New Rules Steps
Use Case
With these enhancements, users are no longer required to execute the rules one batch-at-a-time. This feature automates the Rules process, saving users from having to spend significant time executing and monitoring the lengthy process.
Description
These enhancements to the Load workflow automate the rules steps of the migration process.
Enablement & Configuration
This feature is automatically enabled. Migration users have the option to skip these optional steps.
Notify Users after Step Completion
Use Case
Users need to be notified after any of the steps are complete so that they know when and if the next step can begin.
Description
This feature introduces two new Notifications that are sent to Users after a Step has completed. One notification utilizes the system’s Notification Service, while the other one involves informing the user by email.
Enablement & Configuration
This is automatically enabled and can’t be disabled.
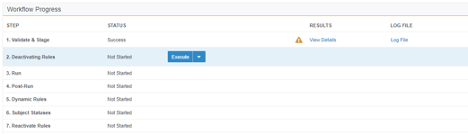
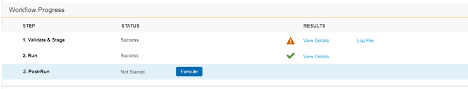
Migration: UI Redesign - Validate & Stage, Run, Post-Run
Use Case
This design makes the Load workflow more intuitive and makes the UI more user-friendly. Users can quickly review load progress with fewer clicks in the new Workflow Progress table.
Description
This release introduces a new design to make the workflow more intuitive and the UI more user-friendly. This feature focuses on the design of existing load workflow steps: Validate & Stage, Run, and Post-Run. Part of this design is the Workflow Progress table. This table does the following:
- Walks users through each Step of the Load workflow
- Provides controls to execute or skip each Step
- Provides a consistent user experience when viewing the results of completed Steps
- Provides quicker access and greater detail for any errors and warnings that may occur during a Step
This feature removes the extraneous Run step from the Load workflow, simplifying the Migration workflow.
Enablement & Configuration
This feature is automatically enabled.
UI Redesign: Validate & Stage, Run, Post-Run
Use Case
This design makes the Load workflow more intuitive and makes the UI more user-friendly. Users can quickly review load progress with fewer clicks in the new Workflow Progress table.
Description
This release introduces a new design to make the workflow more intuitive and the UI more user-friendly. This feature focuses on the design of existing load workflow steps: Validate & Stage, Run, and Post Run. Part of this design is the Workflow Progress table. This table does the following:
- Walks users through each Step of the Load workflow
- Provides controls to execute or skip each Step
- Provides a consistent user experience when viewing the results of completed Steps
- Provides quicker access and greater detail for any errors and warnings that may occur during a Step
This feature removes the extraneous “Run” step from the Load workflow, simplifying the Migration workflow.
Enablement & Configuration
This feature is automatically enabled.