New Features in 18R1.4: Derived Item Values, Main Study Extract, and more...
Release Date: July 13, 2018Release Date: July 13, 2018
We are pleased to bring you the following new features in this week’s release. See details about each feature’s enablement below.
Monitoring & Data Management
New Standard Template Reports
Study administrators and data managers can now view the following report templates in the Reports tab:
- Query Aging By Site: Summary report of query counts by query age grouping (<1 Day, 1-3 Days, 3-5 Days, 5-7 Days, >7 Days) grouped by study site.
- Subject Progression: Detailed listing of event completion grouped by study site and subject.
- Form SDV Monitoring Activity per Week: Summary report of form SDV monitoring activity per week grouped by site.
- Comprehensive Form Summary: Detailed listing of submitted forms grouped by site and subject.
Event Operational Summary Report Templates
Study administrators and clinical programmers can now create reports using the Template: Event with Event Operational Summary and Template: Event Operational Summary report types. This release also includes two standard report templates using these types: Standard Template: Overdue Form Entry per Event (summary of Events with overdue data entry tasks, including estimated Form workload) and Standard Template: Schedule Deviation Report (summary of Study Site and Subject adherence to the planned visit schedule per Study protocol). Using these report types and templates allows users to create reports based on the following information about scheduled Events: Event Window Status (In Range, Early, Late), Days Outside Event Window, Expected Event Form Count, and Form Entry Overdue Days.
Views & Clinical Data Mapping
Availability: In the current release, this area of the application is only available to Veeva Services.
Enhanced Views Editor for Clinical Data Mapping
The new Views Editor feature enables clinical programmers to more quickly and easily construct clinical data export View Definitions in Studio. Using this feature, the clinical programmer can now map data points, data point metadata, static values, and formula-derived values to a specified set of Column Definitions.
The Views Editor also introduces the following view-related enhancements:
- A Properties panel for View Definitions
- Delete existing View Definitions
- Create copies of existing View Definitions using a Save As button
Automatic Creation of Study Data Extraction View Set
Study administrators and clinical programmers can now auto-generate a View Set Definition containing a View Definition _for each _Form Definition in the Study. Each column on a form’s view maps to an Item Definition referenced in the study’s Casebook Definition. The main study extract View Set includes a full clinical data dump containing all data points collected within the Study.
Study Design & Studio
Availability: In the current release, this area of the application is only available to Veeva Services.
Derive Item Values via Rules
With this release, clinical programmers can now create rules to set a value on an Item. Clinical programmers first set the Item Type to Derived, and then write a rule to set the value. Derived Item actions are not available for cross-form rules, and they are limited to identifiers on the same Form for both the expression and the derived Item. With derived Item values, data entry users no longer need to calculate certain values independently, saving time and reducing errors. Note: In this release, Set Item Value-type rules are single-_Form_. Any Items you reference in the expression must be on the same Form as the derived Item.
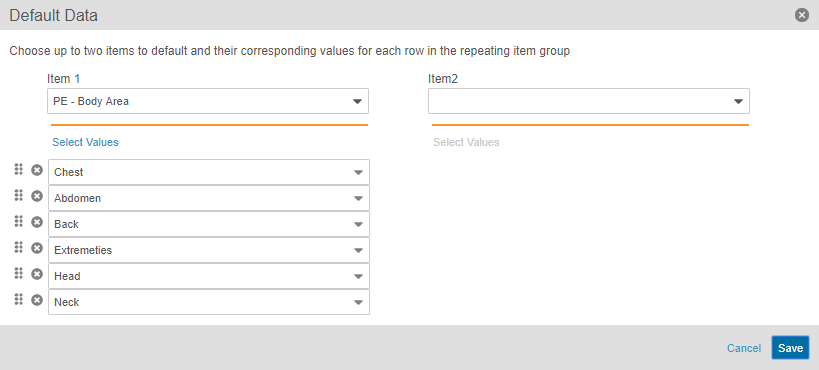
Automatic Creation of Chosen Repeating Item Groups
Study designers can configure repeating Item Group Definition so that when a data entry user creates a new Form, the system creates the configured Item Groups automatically, including setting the configured default values on the new Items. When adding an item group to a form in Studio’s Design view, Studio users can configure the Default Data property, selecting two codelist-type Item Definitions to drive the creation of new Item Groups. For example, if five specific body system exams need collection for a Medical History form, Vault EDC creates those five Item Groups (one per exam) upon creation of the Medical History form, and then populates the Body System field on each Item Group with a body system. Default repeating Item Group records count against the Repeat Maximum set for the Item Group.
Dynamic Fields for Repeating Item Groups
Veeva Vault EDC now supports the dynamic enablement and disablement of only dependent Item fields in a repeating Item Group with the same Sequence Number as the controlling Item. There are scenarios where a field should be enabled or disabled based on an Item field value in the same repeating Item Group sequence. For example, a Study may have a Physical Exam repeating Item Group with three Items: Physical Exam Test, Physical Exam Results, and Physical Exam Abnormal Description. A clinical programmer can create a Skip Item type rule so that Physical Exam Abnormal Description item only enabled when the user selects Abnormal for the Physical Exam Results item in that sequence. The selection would only enable that Item for the current sequence.
| Item Group Sequence # | Physical Exam Test | Physical Exam Results | Physical Exam Abnormal Description |
|---|---|---|---|
| 1 | Heart | Normal | Item Entry Disabled |
| 2 | Lungs | Abnormal | Item Entry Enabled |
| 3 | Skin | Normal | Item Entry Disabled |
When the controlling Item is part of a non-repeating Item Group, and the dependent Item is part of a repeating Item Group, then the dependent Item will be enabled or disabled in all Item Group sequence numbers that exist.
In the current release, this feature does not support the following scenarios:
- Controlling and dependent Items on different repeating Item Groups
- A controlling Item in a repeating Item Group and a dependent Item in a non-repeating Item Group
Validate Event Window Settings
Vault EDC now includes event window settings in publishing validation. Vault EDC now provides warnings for checking the first Event, Events not in the Event Schedule, and invalid Offset Events. This enhancement limits opportunity for errors surrounding event windows.
Remove Component Study Selection
With this release, we removed the ability to add objects from another Study in Studio’s Design view.
Study Administration & Study Tools
Availability: In the current release, this area of the application is only available to Veeva Services.
Renamed Jobs & History Subtabs
With this release, we renamed the Jobs subtab in Study Tools > Jobs to Job Schedule. We also renamed the History subtab to Job History. The + New Job button now displays on both tabs. This name change clarifies the purpose of each subtab.
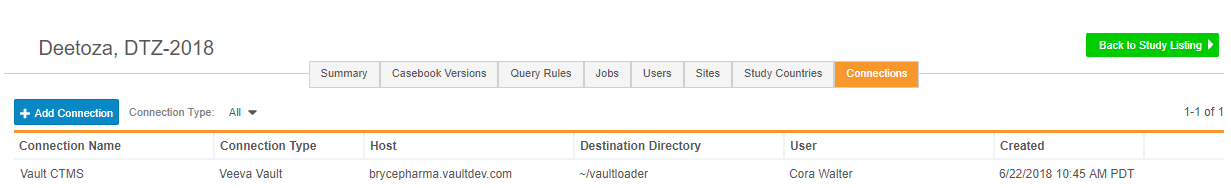
Export Files to Configurable FTP Location
This feature provides study administrators with the ability to add a new FTP connection, so that Vault EDC can send clinical data securely to a chosen location. Vault EDC supports SFTP (Secure File Transfer Protocol) and FTPS (File Transfer Protocol). Study administrators can use FTP connections to send data to other Vault applications, such as Vault CTMS or Vault eTMF. Study administrators can view existing and add new connections from Study Tools > Connections.
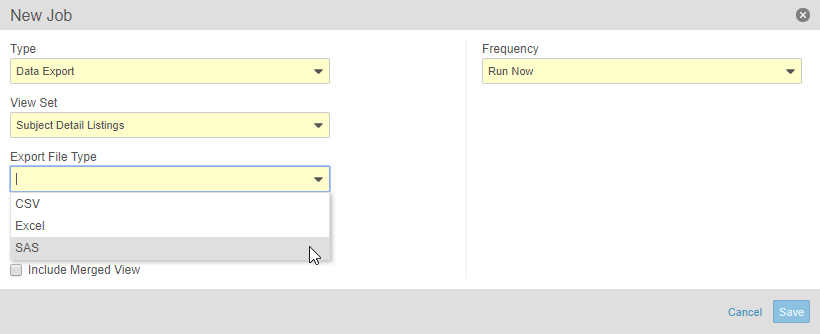
Clinical Data Export in SAS Format
This feature adds a new option to the Data Export job in Study Tools. Study administrators can create a job to export data, using a View Set, and convert that data into SAS format (including the XPT file). Study administrators can also download that file from Study Tools > Jobs > History. Study administrators can also send the SAS file securely to a chosen, external location via FTP (File Transfer Protocol) using the FTP Connections feature.
Export Study Users into CSV
Study administrators and data managers can now export a list of Users in their Study from Study Tools > Users to a CSV spreadsheet. If the study administrator applies any filters to the Users listing, the CSV export includes only the filtered Users. This feature supports the inventory efforts of data managers and others responsible for user management.
Subject Milestones & Veeva CTMS Connection
With this release, clinical programmers can now define subject milestone exports in Vault EDC that update subject information in an associated CTMS vault for use with operational reporting. The export from Vault EDC contains the following subject information: Study, Study Country, Study Site, Subject ID, Subject Status, Screened Date, Screen Failed Date, Randomized Date, Enrolled Date, Withdrawn Date, End of Treatment Date, End of Study Date. Users with access to both the EDC and CTMS vaults can open a subject’s Casebook in the EDC vault from their CTMS vault.
Feature Enablement Details
| Feature | Enablement |
|---|---|
| Monitoring & Data Management | |
| New Standard Template Reports | Auto-on |
| Event Operational Summary Report Templates | Auto-on |
| Study Design & Studio | |
| Internal Only: In the current release, this area of the application is available only to Veeva Services. | |
| Derived Item Values | Support |
| Default Repeating Item Group Records | Configuration 3 |
| Dynamic Fields for Repeating Item Groups | Configuration 4 |
| Validate Event Window Settings | Auto-on |
| Remove Component Study Selection | Auto-on |
| Views & Clinical Data Mapping | |
| Internal Only: In the current release, this area of the application is available only to Veeva Services. | |
| Enhanced Views Editor for Clinical Data Mapping | Auto-on |
| Automatic Creation of Study Data Extraction View Set | Auto-on |
| Study Administration & Study Tools | |
| Internal Only: In the current release, this area of the application is available only to Veeva Services. | |
| Renamed Jobs & History Subtabs | Auto-on |
| Export Files to Configurable FTP Location | Auto-on |
| Clinical Data Export in SAS Format | Auto-on |
| Export Study Users into CSV | Auto-on |
| Subject Milestones & Veeva CTMS Connection | Configuration |
| Enablement Notes | |
|
1 This feature was postponed to a later release. 2 In vaults where Specialized Application Area for Site Review & Monitoring is enabled. 3 While the settings for this feature display automatically in Studio, a clinical programmer must configure this as part of their study design before this feature will display in Vault EDC's data entry area. 4 While the settings for this feature display automatically in Studio, a clinical programmer must configure Skip Item-type rules on repeating Item Groups before this feature will affect Vault EDC's data entry area. |
|
Enablement Legend
See the following explanations for feature enablement options:
| Enablement | Description |
|---|---|
| Auto-on | Automatically activated and no configuration is required before using the feature; note that in some cases, a new feature is dependent on another feature that must be enabled or configured. |
| Admin Checkbox | Admins must turn on the feature with an Admin checkbox. Note that some “Auto-On” features have a checkbox setting that hides the feature; these will show “Auto-On.” |
| Configuration | Admins must configure the feature (separately from an Admin checkbox) before it is available to use or is active; for example, a Clinical Programmer must create an Item Definition of a certain new Item Type. |
| Support | On/off option controlled by Support. |