New Features in 17R3.5: Casebook Validations and more...
Release Date: March 2, 2018Release Date: April 6, 2018
We are pleased to bring you the following new features in this week’s release. See details about each feature’s enablement below.
EDC
Radio Buttons
Vault EDC now supports the ability to configure an Item field on a Form as a radio button. Users can configure radio buttons to display either vertically or horizontally. When an Item field configured as a radio button appears in the same row as another Item field on a Form, it will always display vertically, even if the Item Definition is set to display horizontally.
Data Management Review
With this feature, data management teams can flag an Item field as reviewed with the Data Management Review (DMR) flag. DMR replaces source data review (SDR) throughout Vault EDC. Users with the appropriate permissions can perform DMR at the Item, Item Group, and Form level. Users can utilize Review Plans to determine which Item fields require DMR. In a DMR Review Plan, Item fields can be set to Required, Optional, or Not Available. All Item fields on a submitted Form that have DMR set to required and are incomplete appear in the task bar.
EDC Studio
Detect Duplicate Objects
When an object from another Study is reused more than once, and then versioned in the current Study, Vault can include both instances of the object (the original and the new version) in the ODM XML export. Vault can now detect when this occurs and prompts users to copy or reuse the existing object in the current Study. This saves clinical programmers time by preventing the creation of unnecessary or duplicate records at import.
Delete Casebook Object Records from Studio
Studio users can now delete object records without leaving the Studio tab. When an object record is not in use, a user can delete the record immediately. If an object record is referenced in the study design, users can remove any object relationships, and then delete the record. Users cannot delete the record if its related execution record has user-entered data. With this feature, Studio users can easily remove orphan objects from the casebook version in Studio, instead of from Admin > Business Admin.
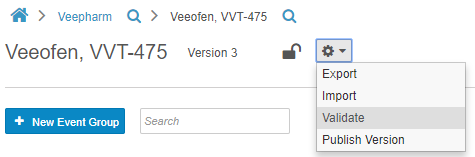
Casebook Validations
Before publishing a casebook version, Studio users can run a report to validate the study, checking for issues with the study and rule designs. This report identifies deviations that will cause issues when importing the Casebook Definition into other study environments. The report generates a list of errors and warnings that a Studio user must resolve to import the design without errors. Studio users also have the option to initiate validation on their casebook version at any time, by choosing Validate from Studio’s Actions menu. This option initiates a job. When complete, Vault sends the user a notification email with a link to download the validation results. With this feature, users no longer need to manually review and identify design deviations that may cause errors.
Clinical Data Mapping
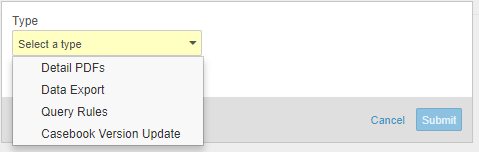
Clinical programmers can now use Studio to build and manage mapping between study data and export-ready views of that data. Data managers and other study administrators can then use Study Tools to create data exports based on these views. In Studio’s Browse view, users can navigate to the View tab to create views, defining what each view contains and their formatting. Once a clinical programmer creates a view, a data manager can initiate a Data Export job from Study Tools > Jobs to export data into a CSV, with scope and formatting defined by the chosen View.
Unit Versioning
Vault now versions Unit Definition and Unit Item Definition object records with each version of a Casebook Definition. When a Studio user creates a new casebook version, Vault automatically creates relationships for each unit and unit item as part of version creation. When a Studio user makes changes to a unit or unit item, Vault creates a new version of the unit or unit item to associate with the casebook version, and then updates the relevant relationships. With this feature, Studio users are now also able to copy units during study design.
Updated Import UI in Studio
With this release, we updated the UI for the Import dialog in Studio. The dialog is now easier to use and more similar to other existing dialogs.
EDC Study Administration
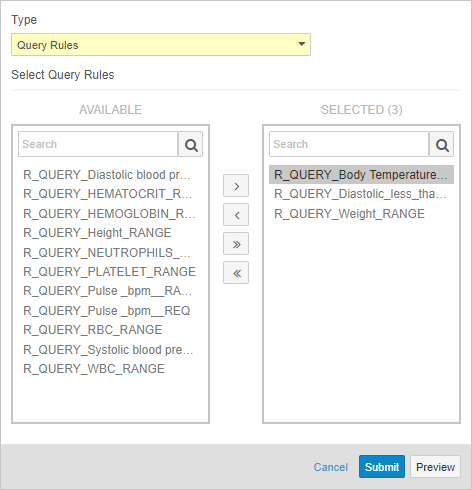
Admin: Query Rules Job Enhancements
Data managers and other study administrators can use Study Tools > Jobs to run a query rule. With this release, data managers can now run a set of rules with a single job. Before running a rule, data managers can run a job to preview of the results before Vault applies the rule evaluation to the affected Items or Events. After previewing, a data manager can easily initiate the another Query Rules job to run the previewed rules. Previewing these results is especially useful when adding a new rule to a study or when changing a rule’s criteria expression. By reviewing a preview before initiating the job, data managers can ensure that there are no unexpected queries.
Admin: Casebook Version Update Job
With this release, data managers can now also initiate a Casebook Version Update job from Study Tools > Jobs. When a site’s casebook version changes, this job updates a site’s subject Casebooks to the new version, starting at the next Event in the casebook (Events prior to the update are not altered). Additionally, if the new version dictates that an additional new Event belongs in the schedule, this job adds such Events to existing subject Casebooks.
Base Standard Template Report Permission Set
With this release, we introduced the Base Standard Template Report Permissions permission set. This permission set includes the following permissions:
- Admin: Page Links: Read
- Application: Dashboards and Reports: Read Dashboards and Reports
- Application: Dashboards and Reports: Create Dashboards
- Application: Dashboards and Reports: Share Dashboards
- Objects: Report: Read, Create, Edit
- Tabs: Reports: View
Vault assigns this permission set to the following security profiles by default:
- EDC Investigator
- EDC CRA
- EDC Lead CRA
- Monitor User
- EDC Data Manager
- EDC Lead Data Manager
Which standard template reports users can view depends on several configuration options. Vault Owners or System Administrators can determine which users can access which reports, based on their role in the vault.
All Countries & All Sites User Permissions
Admins can now configure a single EDC Execution User Role Setup record to provide a user access to all countries in a study or all sites in a study, instead of creating multiple records to provide a user access to individual sites. This feature saves Admins time when onboarding new users and managing the access of existing users.
Vault Objects
User & Person Object
The new User object allows customers to conveniently interact with user data throughout Vault. The User object contains records of all existing Vault users, enabling customers to report on user data, create custom fields, and reference users from documents or objects with lookup fields and reference constraints.
To immediately leverage the full flexibility of Vault objects, we’ve converted existing user reference fields to User object reference fields. For example, the Assigned To field on the Activity object makes this object available as a related list on the User record detail page.
Note that the Created by and Last Modified By fields were not converted to User object reference fields, nor were other document references to user like Checked out by.
The Person object allows both Vault users and non-users to be contained in a single object. When referencing a user from a Person record, Vault synchronizes the standard fields from both records. For example, updating the mobile phone number in a Person record automatically updates the User record.
Vault also synchronizes domain-level attributes on User like username and email across all vaults to which that user is a member. This includes cross-domain vaults.
Behavioral Changes Related to the User Object
The new User object feature includes the following behavioral changes:
- Faceted Search for Migrated User References:Prior to 18R1, all references to users had a single select dropdown UI. After 18R1, all migrated user reference fields will appear as facets like any other object reference.
- Refreshing & Cloning Vaults: When cloning or refreshing a vault, any user references will be replaced with an ID of ‘1’. To support that process, the User object will include a System user record with the assigned ID of ‘1’. Additionally, refreshing a vault will not include any custom fields created on the User object at this time.
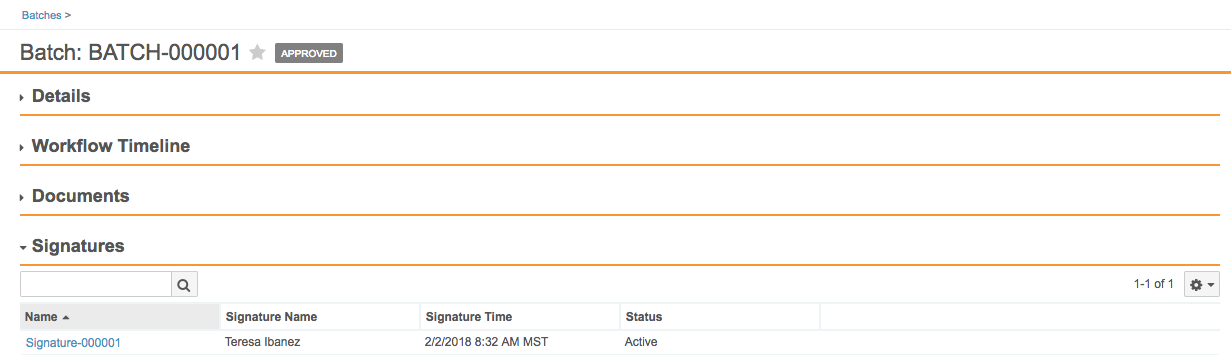
eSignature Management for Objects
Vault now stores eSignatures captured by workflows on object records as related, system-managed object records. With this change, eSignatures display as a related list on a record detail page. This change also allows users to report on eSignatures related to object records.
Note that all existing eSignatures captured through object workflows will automatically migrate to signature objects.
With this enhancement, we’ve also introduced a new object lifecycle state entry action that enables Admins to set current eSignatures to obsolete: Make signature records obsolete. This functionality is useful when a record returns to an earlier state, for example, when a record is rejected and it’s previously captured signatures no longer apply.
Minor Enhancements
Support for DateTime Data Type
With this release, the Vault Formula Expression Grammar for Objects now provides support for the DateTime data type and related functions.
The following configuration options are now available:
- Admins can use DateTime fields as source tokens in Formula Field and Field Default Expressions.
- Formula Fields can have DateTime as return type.
- Admins can configure expression-based default values for DateTime fields.
The following functions are now available for formulas in Vault:
dateTimeDiffdateTimeAdd
Feature Enablement Details
| Feature | Enablement | Application |
|---|---|---|
| EDC | ||
| Radio Buttons | Configuration | EDC |
| Data Management Review | Configuration | EDC |
| EDC Studio | ||
| Detect Duplicate Objects | Auto-On | EDC |
| Delete Casebook Object Records from Studio | Auto-On | EDC |
| Casebook Validations | Auto-On | EDC |
| Clinical Data Mapping | Auto-On | EDC |
| Unit Versioning | Auto-On | EDC |
| Updated Import UI in Studio | Auto-On | EDC |
| EDC Study Administration | ||
| Admin: Query Rules Job Enhancements | Auto-On | EDC |
| Admin: Casebook Version Update Job | Auto-On | EDC |
| Base Standard Template Report Permission Set | Auto-On | EDC |
| All Countries & All Sites User Permissions | Auto-On | EDC |
| Vault Objects | ||
| User & Person Object | Auto-On | Platform |
| eSignature Management for Objects | Auto-On | Platform |
| Minor Enhancements | ||
| Upload Files when Adding Document Relationships | Auto-On | Platform |
| Support for DateTime Data Type | Configuration | All |
| Enablement Notes | ||
| 1 This feature was postponed to a later release. | ||
Enablement Legend
See the following explanations for feature enablement options:
| Enablement | Description |
|---|---|
| Auto-on | Automatically activated and no configuration is required before using the feature; note that in some cases, a new feature is dependent on another feature that must be enabled or configured. |
| Admin Checkbox | Admins must turn on the feature with an Admin checkbox. Note that some “Auto-On” features have a checkbox setting that hides the feature; these will show “Auto-On.” |
| Configuration | Admins must configure the feature (separately from an Admin checkbox) before it is available to use or is active; for example, a Clinical Programmer must create an Item Definition of a certain new Item Type. |
| Support | On/off option controlled by Support. |
Known Issues
Formula Fields
When selecting a field to filter by, Vault shows formula fields as options. Formula fields are not available for search or filters because their values are calculated at run-time.