What's New in 22R2
Pre-Release Date: July 11, 2022 | CDB Pre-Release Date: July 18, 2022 | Release Date: July 29 & August 5, 2022We are pleased to bring you Veeva Clinical Data in 22R2. Read about the new features below. You can find information on enabling new features in the 22R2 Feature Enablement Details. Information on developer features (REST API) is in the Developer Portal.
Clinical Data
Features in this section are changes that apply to all application areas of Veeva EDC and CDB.
Audit Entries for Event Date, Planned Date, & Overdue Date
Use Case
We will now audit date fields with values when they are added to Veeva when data is migrated to EDC from another system.
Description
To support migrated data, audit records for Event Date, Planned Date, and Overdue Date fields will be added when events are created if these fields are created with non-blank values.
Enablement & Configuration
This feature will automatically be available for all studies.
Audit Trail Enhancement: Additional Form Entries
Description
The Form Audit Trail now includes audit entries for changes relating to the following fields:
- Study Country
- Site
Enablement & Configuration
This change applies automatically.
Data Entry
Features in this section are changes to the Data Entry tab, a working area for investigators and clinical research coordinators to enter study execution data.
Lab Header Styling Updates
Use Case
Lab form header fields are now left-aligned, which makes it easier for the user to enter data about the lab and the patient.
Description
With this feature, we’ve updated the look and feel of the header section for lab forms. The fields for Collection Date Time, Lab Location, Age, and Sex will be left-aligned, making it easier to enter data.
Enablement & Configuration
This will be available for all studies using lab forms.
Include Hint Labels in Detail and Closeout PDFs
Use Case
The Detail PDFs and Closeout PDFs will now match how the form looked to the site during data entry.
Description
When a form is configured with a hint label, that hint label will now be included in the Detail PDFs and the Closeout PDFs.
Enablement & Configuration
This functionality will be available by default for studies using hint labels.
New Intentionally Left Blank Icon
Use Case
This provides consistent iconography across the system.
Description
We added a new icon for Intentionally Left Blank in the Actions menu for Forms, Item Groups, and Items.
Enablement & Configuration
This new icon will be available by default.
Data Review
Features in this section are changes to the Review tab, a working area for clinical research associates and data managers, or to review functionality within the Data Entry tab.
Study Summary Metrics Report
Use Case
The new Study Summary Metrics Report and the updated Event Progress Listing will provide a real time summary of SDV and DMR, allowing users to monitor how their study is progressing.
Description
With this new report, CRAs and Data Managers can see a summary of SDV and DMR progress in their study. The Study Summary Metrics Report will detail how many forms, events, subjects, and sites have been reviewed at the Site and Country levels. We’ve also updated the Event Progress Listing job to include new columns for review data, including the number of forms that have completed SDV or DMR at the event and the SDV/DMR status of the event date.
Enablement & Configuration
The Study Summary Metrics Report will be available in the Review tab for studies using Review Rollup V2. Users with View SDV or View DMR and the Manage Jobs permissions will be able to access this new job type. If the user has access to View SDV or View DMR, there will be a new checkbox in the New Job dialog to Include Review Data. When this is selected, the new SDV/DMR columns will appear in the listing. These columns will only populate for studies using Review Rollup V2.
Additive Review Listing
Use Case
With this listing, data managers can review sites with high amounts of additive review or identity users who are performing additive review.
Description
The Additive Review Listing will provide data managers with a report on items additively reviewed in the study. It will include a summary report that gives a high-level view of items that have been additively reviewed by the site. This listing will also include a detailed report at the item or event date level, which includes the following information: the user who performed the additive review, the datetime when the item or event date was reviewed, and the additive reason why.
Enablement & Configuration
This listing will only be available for studies where additive review is enabled in Studio. Users with access to the Review tab, View SDV or View DMR, and the Manage Jobs permission will be able to generate this listing from the Review tab.
Form Link Audit Trail in Review UI
Use Case
The Form Link Audit Trail was previously only available in the Data Entry tab, so CRAs and Data Managers did not have insight into the audit history for Form Linking. With this feature, CRAs can see who linked the forms and when, and they can also observe if the links changed between two forms, such as Adverse Events and Concomitant Medications.
Description
Review users, like CRAs and Data Managers, will now be able to view the audit trail for form links from the Review tab. The Form Link Audit Trail will be available in the form’s More Actions menu if the form is linked to another form. All form link changes will be reflected in the audit trail.
Enablement & Configuration
This feature will only be available for studies using Form Linking. It is available for both Form Linking V1 and V2. The Review user must have the View Form Linking permission in order to view the audit trail.
Protocol Deviation Audit Trail UI Enhancements
Use Case
These changes improve the user experience for viewing audit data.
Description
The audit trail for Protocol Deviations has been updated to be more user-friendly. The audit trail dialog is now responsive to the user’s screen size and can be closed using the “X” or the new Close button. The header of the audit trail grid will also remain visible as the user scrolls to see more audit data.
Enablement & Configuration
This feature is on by default for all studies. These changes will be visible to users with access to view Protocol Deviations.
Prevent Creation of Protocol Deviations in Locked Sites
Use Case
With this enhancement, we’ve improved the messaging for Review users to let them know what’s possible and not possible when a site is locked.
Description
Review users won’t be able to open new Protocol Deviations in locked sites.
Enablement & Configuration
This enhancement will be automatically available for all studies. Users with the Create Protocol Deviations permission will be affected.
Clinical Coding
The following are new features for Veeva Coder, the clinical coding area for Veeva Coder.
Dictionary Search Enhancements
Use Case
Coders will find more relevant dictionary results when searching single characters, special characters, common terms, and irrelevant terms. They can also make use of double quotes for exact match only results.
Description
Coders will be able to find more relevant results when searching phrases that include single characters such as the “E” in “Vitamin E,” phrases with terms that include special characters such as “+immu boost,” and phrases that include popular terms such as “carbonate” in “calcium carbonate.” The Dictionary search can also return more accurate results when the search phrase includes non-relevant terms. Coders can also search for exact match results only with the use of double quotes.
Enablement & Configuration
These enhancements apply automatically.
Assessments
The following are new features for the Assessments area of Veeva EDC.
Medical Assessments: Data Changes & Reassessment
Use Case
With the reassessments feature, assessors can easily identify when assessed data has changed and when a reassessment is required.
Description
Study designers can now configure an Assessment Definition to automatically trigger a reassessment when data from the selected Items is modified post-submission. This configuration occurs in the new Reassessment phase of assessment configuration in Studio > Assessments.
When a site changes the data for one or more of the chosen Items, Vault automatically generates a new Assessment and marks it as a reassessment. The Assessments tab now includes a column for “Reassessment”. In the assessment’s header, Vault also shows the “Yes” or “No” value for Reassessment.
This feature added a new column to the Assessments sheet in the Study Design Specification, “Trigger for Reassessment”. For Items that trigger reassessment, Vault enters “Yes” in this column. For Items that don’t trigger reassessment, Vault enters “No” in this column.
Enablement & Configuration
Reassessment functionality is automatically available, but a study designer must first configure an Assessment to use reassessments before this feature is exposed to assessors.
Lock Assessments when Study or Site is Locked
Use Case
When a site or study is locked, users will not be able to change medical assessment data, similar to how they cannot change clinical data.
Description
Users will be prevented from making changes to medical assessments when a study is locked. If the site is locked, users will be able to complete open assessments but will not be able to modify completed assignments.
Enablement & Configuration
This functionality will be available by default for studies using Medical Assessments.
Study Design & Configuration
Features in this area apply to Studio, the study design and configuration area for Veeva EDC.
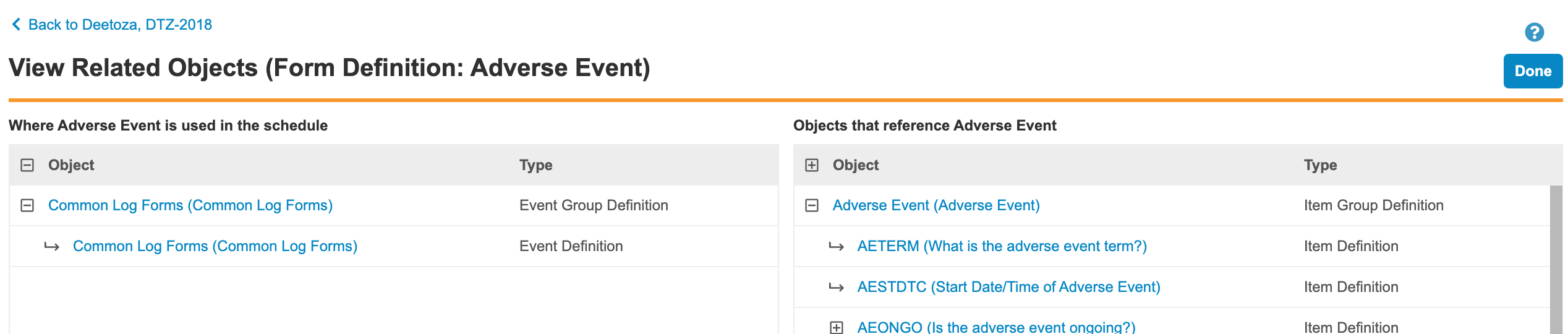
Object Usage Report
Use Case
When editing a definition, users want to know the impact of their changes (either in the schedule, not used in the schedule, or, due to reuse, the impact of that change on other definitions where that object is used).
Description
The Object Usage Report provides a method for reviewing where a definition (object) is in use and what other definitions are related to it. With this report, users have the ability to review the potential impact of changes and to navigate up and down the hierarchy of definitions.
This report is accessed via the View Related Objects action in the Actions menu for Event Groups, Events, Forms, Item Groups, Items, Codelists, and Units.
Users can navigate through the following associated objects:
- Event Group
- Event
- Form
- Item Group
- Item
- Codelist
- Unit
In addition, users can see the following related objects:
- Rules
- Form Links
- Item Form Links
- Assessments
- Review Plans
- Coding Configurations
The object usage report doesn’t cover the following:
- Subject Groups
- Lab Panels
- Views
- Protocol Deviations
Enablement & Configuration
This feature is automatically available.
Updated Properties Panel
Use Case
These were aesthetic changes to alight the look and feel across the application.
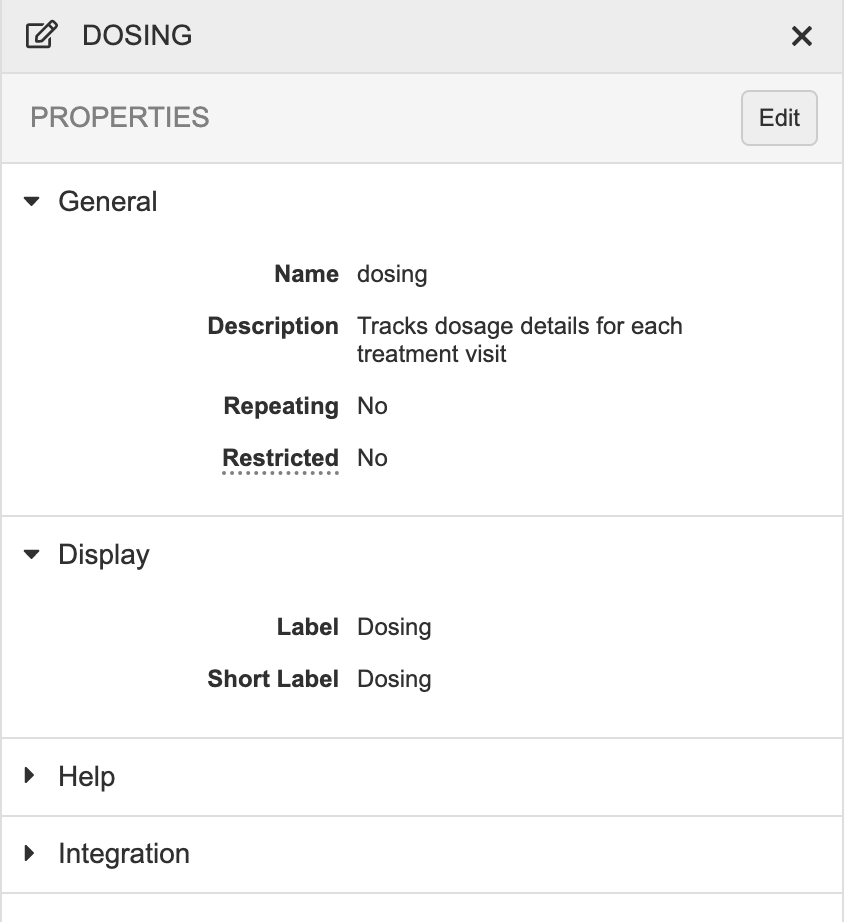
Description
With this release, we updated the styling of the Properties panel to match our new application standards. This included moving actions to a row-level Actions menu (for list views) or near the Edit button (for design views).
Vault no longer displays the Properties panel for Codelists, Units, or Rules, as these objects have their own editors.
As part of this enhancement, we removed the “Mandatory” field from the properties for Events and Forms, as this property was unused.
Enablement & Configuration
This feature is automatically available.
Max Length for Codelists
Use Case
This feature allows study data extracts and CDB to have the correct field length for SAS definitions.
Description
Study designers can now set the Length for codelist-type Items, which allows study data extracts and CDB to have the correct field length for SAS definitions. Prior to this release, users couldn’t edit this property for codelist-type Items. The default is 1500.
Enablement & Configuration
This feature is automatically available.
Updated Handling for Dynamic Event Group Deletion
Description
With this release, Vault no longer deletes a dynamic Event Group when the only dynamic Event present in it is deleted.
Enablement & Configuration
This change applies automatically.
Dynamic Rule Enhancement: Better Support for PPC Changes
Description
Vault now ignores the results of inactive dynamic rule definitions. If a rule is marked as Inactive after it has been run, and another dynamic rule attempts to add or remove the same targeted objects, Vault is now able to execute the new rule without interference from the inactive rule’s results.
Study designers should be aware of this change, but there is no visible change or action required. This enhancement helps improve performance and prevent issues during rule execution.
Enablement & Configuration
This change applies automatically.
Study Administration
Features in this section apply to System Tools or EDC Tools, a study-level administration area for Veeva EDC.
New Navigation Pattern for EDC Tools
Use Case
Previously, EDC Tools used a layout distinct from other CDMS components. These enhancements unify the look and feel of the application, and make it easier to navigate and perform critical study configuration processes.
Description
With this release, we introduced a navigation pattern and UI of EDC Tools pages to provide an exceptional user experience. These changes include:
- Moving all tabs to a left navigation panel
- Providing study instance environment type, build version, and lock status throughout EDC Tools
- Tab stickiness when navigating away and back to EDC Tools
- Enhanced layout of the Jobs, Casebook Versions, Review Plans and Rules subtabs
Enablement & Configuration
The new navigation pattern is automatically enabled.
Amendment Preview: Copy Study Data
Use Case
This feature allows lead data managers the opportunity to preview the result of an amendment on actual study data.
Description
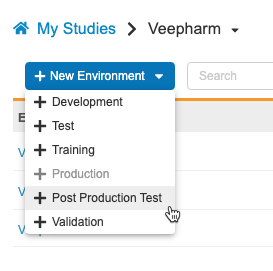
Study data, like execution data and restricted data if applicable, can be copied from Production. This does not include CDB or custom object data. Data copied to the Post Production Test (PPT) environment cannot be merged; subsequent Copy Study Data actions will first remove any existing data.
As part of this feature, we renamed the UAT environment type as TEST. Existing Environments will continue to have “_UAT” appended, but new Environments will have “_TST”. The new environment action is now labeled + Test.
Enablement & Configuration
Contact Veeva Support to enable this feature.
Learn More
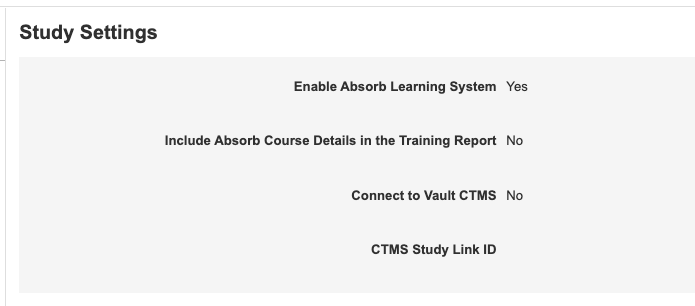
Study Settings in EDC Tools
Use Case
With this change, lead data managers and study designers can manage study settings that were previously only accessible from Admin > Business Admin. Bringing this functionality into the familiar EDC Tools tab provides enhanced usability and speed.
Description
With this release, we added a new landing page in EDC Tools, Study Settings, where users can manage the following study-level settings:
- Enable Absorb Learning System
- Include Absorb Course Details in the Training Report
- Connect to Vault CDMS
- CTMS Study Link ID
The ability to manage study settings is controlled by the new Manage Study Settings permission, which is assigned to the following standard Study Roles:
- CDMS Super User
- CDMS Lead Data Manager
- CDMS Librarian
- CDMS Study Designer
- CDMS User Administrator
Enablement & Configuration
This feature is available automatically.
Audit Trail Export to Include Assessments & Protocol Deviations
Use Case
Previously, the audit trails for Assessments and Protocol Deviations were only available in the Medical Assessment and Protocol Deviation modules. They can now be exported, alongside other clinical data audit history, in the Audit Trail Export.
Description
The Audit Trail Export will now include audit entries for Medical Assessments and Protocol Deviations.
Enablement & Configuration
This feature will automatically be available for all studies using Medical Assessments or Protocol Deviations.
Event & Event Date Columns Added to Item Linking Dialog
Use Case
This enhancement will make it easier to link items to forms at a scheduled event, as users will now be able to differentiate between different events when establishing the link.
Description
When linking an item to a form at a scheduled event, the Item Form Linking dialog will now include columns for the Event and Event Date.
Enablement & Configuration
This enhancement will automatically be available for all studies using item form linking.
Form Audit Event for Updates to a Signature Definition
Use Case
This enhancement adds parity with Data Model 1 studies and lets users know why the form was unsigned.
Description
When a signature’s Legal Reason definition is updated in Studio, an audit entry will be added for any forms that were signed with the previous legal reason. The entry will state that the signature was invalidated because of a change in attestation statement.
Enablement & Configuration
This feature will automatically be available for Data Model 2 studies.
Rename SDV/DMR Columns in Form Progress Listing
Use Case
With this change, it’ll be easier to understand which forms have been fully SDV’d or fully DMR’d.
Description
In the Form Progress Listing, the column headers for SDV and DMR have been renamed to SDV Complete and DMR Complete to more accurately describe the data in these columns.
Enablement & Configuration
This change will automatically be available in the Form Progress Listing.
Include Randomization in Job History - Study Data Extracts
Use Case
Provides more detailed information about the options selected when running the SDE.
Description
When a user selects “Include Randomization Treatment” in the SDE for versions 22R1 and later, that information will be displayed in the information icon in the Job History tab. That information will also be displayed for scheduled jobs if they schedule an SDE with that option.
Enablement & Configuration
This will be displayed if users have the proper permissions (if the Study is unmasked, or if the Study is masked, the user running the job must have the View Unmasked Data permission) to view the “Include Randomization Treatment” checkbox in the SDE job dialog for versions 22R1 and later.
Warn when Locking a Site with Open Assessments
Use Case
When a site is locked, assessors will still be able to complete open assessments, but they will not be able to make any changes to completed assessments. With this enhancement, Lead Data Managers or users who are locking the site will be alerted if the assessors still have work to do, in case they want to wait to lock the site until all work is completed.
Description
Users will be shown a warning message when attempting to lock sites with open assessments.
Enablement & Configuration
This enhancement will be available for studies using Medical Assessments.
New Job Dialog Enhancements
Use Case
This feature will improve the user experience when running or scheduling jobs.
Description
With this release, we’ve made a few small enhancements to the New Job dialog in Review and EDC Tools. Users will no longer be able to change the scheduled job type for scheduled jobs and must delete the scheduled job and create a new one to change the job type. For consistency, we’ve also updated the radio buttons for several of the jobs’ options to match those used elsewhere in EDC Tools. Finally, we’ve removed the “Restore Defaults” button for the following jobs that include the shuttle selector for job options:
- SDE (EDC Tools)
- Core Listings (Review & EDC Tools)
- Event Progress Listing (Review)
- DMR Re-Assignment (EDC Tools)
- Detail PDF (Review & EDC Tools)
- Form Progress Listing (Review)
- Query Detail Listing (Review)
- Reconstitute Code Requests (EDC Tools)
- Subject Progress Listing (Review)
- SDV Re-Assignment (EDC Tools)
Enablement & Configuration
This feature will automatically be available.
Recalculate Planned Date during Retrospective Amendments
Use Case
Planned dates previously were not recalculated during a retrospective amendment. With this feature, planned dates will be recalculated and reflected in EDC.
Description
When an event’s planned dates are added or updated in Studio, the planned dates for this event will be recalculated in EDC for all selected events during the retrospective amendment.
Enablement & Configuration
This feature will automatically be available for all studies.
Warn Users About Restricted Data when Generating a Closeout PDF
Use Case
This enhancement will let users know that the files they’re generating will be marked as restricted and that PIs will also need the restricted data permission in order to download and accept the files in EDC.
Description
When users with Restricted Data Access are generating Closeout PDFs in EDC Tools, they will see a new warning that they are generating restricted PDFs.
Enablement & Configuration
This enhancement will automatically be available. Users with the Lead Data Manager standard role will see this message when generating Closeout PDFs.
Study Data Extract to Include Form Link Items
Use Case
This feature will easily allow users to review Item Form Links in the Study Data Extract alongside form to form links. By including the unique Link ID, users can cross reference an Item Form Link between the clinical dataset and the SYS_LINKS dataset.
Description
In the 22R2 Version of the Study Data Extract, the SYS_LINKS dataset will include data about Item to Form Links. When an item is linked to a form, there will be two rows in the SYS_LINKS dataset to represent the link. One row will represent the item side of the link and the other row will represent the form side of the link. SYS_LINKS will also include information about the form link item, including Item Group, Item Group Definition, Item Group Sequence, Item, and Item Definition for the item row and information about the linked form in the form row. Both will include the link’s unique Link ID.
The Clinical Datasets and Core Listings will now also include the unique Link ID for Item Form Links.
Enablement & Configuration
This feature will only be available in the 22R2 Version of the SDE. The five new columns for Item Groups and items will only be available for studies configured with Item Form Linking.
Study Data Extract: Data Type & SAS Type Changes in the 22R2 Version
Description
In the 22R2 Version of the Study Data Extract, the following changes were made to fix incorrect data types:
- The column CASEBDEF has a SAS Type of numeric in the SYS_EVT, SYS_SITE, and SYS_SUB datasets.
- The column MANUAL has a Data Type of boolean in the SYS_Q dataset.
- The column NSUBMITS has a Data Type of integer and a SAS Type of numeric in the SYS_ASM dataset.
- The column IGSEQ has a Data Type of integer and a SAS Type of numeric, the column INACBYSYS has a Data Type of boolean, and the column LASTINACDT has a Data Type of datetime and a SAS Type of numeric in the SYS_PD dataset.
- The column LABMODIFIER has a Data Type of boolean in the SYS_LABRANGES dataset.
- The columns LABMODIFIER and LABSYSMANAGED have Data Types of boolean, and the column LABPREC has a Data Type of integer and a SAS Type of numeric in SYS_ANALYTES
Enablement & Configuration
This change applies automatically to the 22R2 version of the Study Data Extract job output.
Labs
Features in this section are new features for the Labs module of Veeva EDC.
Versionless Codelist & Unit Definitions for Labs
Use Case
Lab units and codelists no longer need to be synced with Studio, and they can instead be managed directly from the Lab module. This avoids unnecessary deployments and retrospective amendments due to lab unit updates, which causes an artificial increase of caseboooks.
Description
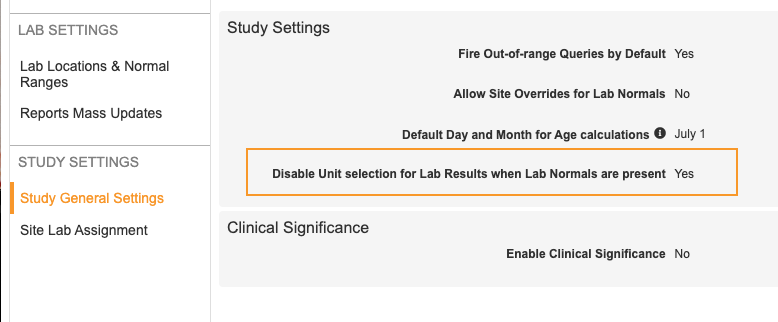
Lab Units and Lab Codelists are now managed solely in the Local Labs module (Labs tab). Changes to the Standard Unit, Conversion, and increases to Precision and Length are allowed after deployment to production (post go live).
As part of this feature, we added the ability to disable unit selection for lab results when normals aren’t present. The Disable Unit Selection for Lab Results setting is available in Labs > Study General Settings for study-level configuration, and in Admin > Business Admin > Vault Configurations for vault-level configuration. By default, this is set to No. When set to Yes, sites can’t change the Lab Result Unit in Data Entry. When set to No, sites can change the Lab Result Unit.
Enablement & Configuration
This feature is automatically available for new Studies. Existing Studies must undergo a migration process to use this feature. Studies must be on Data Model V2 before the Lab Migration can occur.
Role Management & Security
Features in this section are enhancements to the System Tools > Role Management and System Tools > Users areas, as well as changes to standard Study Roles, security, and access control in Veeva Clinical Data.
Study Role Enhancements
Description
With this release, we made the following changes to standard Study Roles:
- Assigned the new Copy Study Data to PPT permission, which controls the ability to copy study data into PPT environments, to the CDMS Super User role
- Assigned the new Manage Study Settings permission, which controls the ability to edit study settings from EDC Tools > Study Settings, to the CDMS Super User, CDMS Lead Data Manager, CDMS Study Designer, CDMS Librarian, and CDMS User Administrator roles
- Assigned the View Study Design and View Library permissions to the CDMS API Read Only and CDMS API Read Write roles
- Assigned the Configure Queries permission to the CDMS Super User study role to support a feature in a future release
Enablement & Configuration
These changes apply automatically to standard Study Roles.
Additional Fields Available in System Tools > Users
Use Case
User administrators can now edit these fields from System Tools > Users, instead of needing to modify these fields from Admin > Users & Groups.
Description
The following fields are now available for editing in System Tools > Users: Domain Admin, Service Availability Notifications, Product Announcement Emails, and Status. These fields can also be modified via user import.
Enablement & Configuration
This feature is automatically enabled.
Course Status in Training Report
Use Case
The existing User Training Report did not adequately delineate between curricula that were 100% complete, curricula that had a new course added, or curricula where a course was split. This new column provides more precision in evaluating a user’s training status and furnishes a clear verification of study access eligibility for audit purposes.
Description
With this release, we provide more clarity in the User Training Report by adding a new column for the user’s course status. The Course Status column will have the following values depending on the relevant scenario:
- Completed
- Optional, Not Completed
- Not Completed
- Previous Course Completed
- New Course Detected
Enablement & Configuration
This feature is only available to certain early adopters. Contact your Veeva Services representative for details.
My Training Tab
Use Case
Previously, a user’s training requirements, training status, and study access could be unclear without simultaneously inspecting the external training system transcript. In addition, some site users may have seen a vague error message when accessing the platform before all training requirements were met. The addition of the My Training Tab eliminates these ambiguities by providing a central location to view and manage training and study access.
Description
With this release, we introduced a new main navigation tab, My Training, that provides applicable users a convenient and straightforward way to manage their training requirements and study access. From the My Training tab, users can quickly determine whether they have outstanding training for a given role, access the learning portal in a new tab, and fetch their individual status from the learning portal in real time.
Enablement & Configuration
This feature is enabled automatically in Vaults using the integration.
Deployments
Features in this section are enhancements to deployment functionality in Veeva Clinical Data.
Prevent Deployment of High Volume Object Configuration
Use Case
This prevents the possibility of errors or other difficulties when deploying user-defined objects that were configured as high-volume.
Description
With this release, we have made vault-level deployments more robust by excluding the deployment of custom High Volume Objects (HVO) which would have caused deployment failures if included in the past.
In previous versions, deployment administrators and other administration roles may have encountered difficulties when deploying user-defined objects that are configured as HVO. With this version, we removed ambiguity and potential for deployment failures by omitting the option to select an HVO in the deployment list.
Enablement & Configuration
This change applies automatically.
Connections & Integrations
Features in this section are new connections or integrations with Veeva Clinical Data or enhancements to existing ones.
Safety Link: Updates Labeled as "Follow-Ups"
Use Case
While safety systems are correctly processing follow ups from Safety Link, Safety Link now correctly labels a Follow-Up as a “Follow-Up” and not as an “Amendment”.
Description
With this release, all follow ups generated by Safety Link are labeled as “Follow-Up”, instead of “Amendment”. This label is used in reports. In the E2B ID C.1.11.1 “Report Nullification / Amendment”, is now null, where previously it used the value “2”, which is reserved for amendments.
Enablement & Configuration
This change applies automatically.
Safety Link: E.i.9 Country, Where the Event Occurred
Use Case
When an SAE occurred outside of the site’s country, Safety Link can now automate the reporting of this site-reported value.
Description
The E2B ID E.i.9 ‘Identification of the Country Where the Reaction / Event Occurred’ is now mappable by Safety Link.
Enablement & Configuration
E.i.9 is automatically available for mapping in EDC Tools.
Vault Clinical Operations to CDMS Connection: Arms & Cohorts (Clinical Operations)
Description
This is a Vault Clinical Operations feature, so this feature is listed in the 22R2 Supplemental Release Impact Assessment instead of the CDMS impact assessment.
This feature contains enhancements to the CDMS & Clinical Operations connector for transferring subjects’ arms and cohort assignments.
Enablement & Configuration
This change is enabled by a Vault Administrator in CTMS.
Vault Clinical Operations to CDMS Connection: Restricted Data (Clinical Operations)
Description
This is a Vault Clinical Operations feature, so this feature is listed in the 22R2 Supplemental Release Impact Assessment instead of the CDMS impact assessment.
This feature enhances the ClinOps to CDMS Connection, making restricted data for subject visits and protocol deviations identifiable when transferred from CDMS to CTMS, and CTMS can control access to restricted data through permissions.
Enablement & Configuration
This change is enabled by a Vault Administrator in CTMS.
Clinical DataBase (CDB) & EDC Clinical Reporting
The following are new features for the Veeva CDB application, EDC Clinical Reporting (the Veeva Clinical Data solution for data cleaning and reporting), or both.
Availability: Clinical DataBase (CDB) is only available to CDB license holders. Contact your Veeva Services representative for details.
Export Package Blinding
Use Case
This feature increases flexibility in which type of user can schedule export package generation. For example, an unblinded user can now schedule the delivery of an unblinding package to an FTP server.
Description
Prior to this release, the blinding of an export package upon delivery or download was based on whether the user had the Restricted Data Access permission. With this release, package blinding is user-defined in the properties of the Export Definition. The default setting is Blinded. A user who has edit access on the Export Definition (the owner of the Export Definition or a vault owner) can change this setting to Unblinded. At package generation, CDB uses the blinding setting to determine blinding. If a package is blinded, users without restricted data access will be unable to download it.
Enablement & Configuration
This feature is enabled automatically. CDB sets any Export Definitions created prior to this release as blinded. To generate unblinded packages for these definitions going forward, a user with edit access should update it to Unblinded in the properties. Export packages generated prior to this release will maintain their existing behavior (blinding is determined by the user’s access to restricted data).
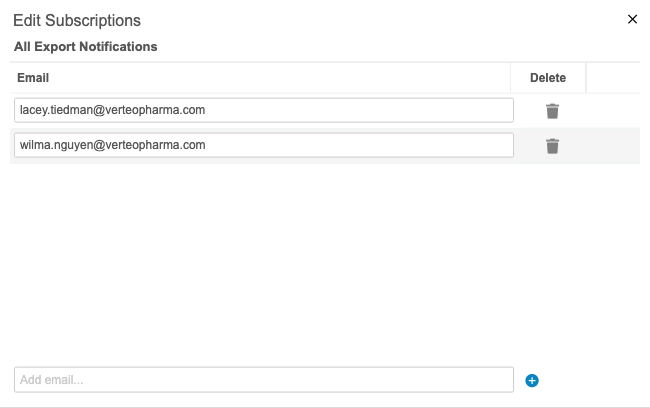
Export Package Notifications
Use Case
This feature improves monitoring for exports in CDB. Export users have insight into the export package metrics. Users can keep track of packages without navigating to the CDB Exports page.
Description
Users can receive notifications about export packages. For example, they can choose to receive an email when a package fails to generate or delivery to an FTP destination fails. An admin user (a user with the View Admin permission) can add users as subscribers to different statuses from Admin > Export Notifications. The following status options are available for notification subscriptions: All Statuses, Package Complete, Package Error, Package Delivered, and Package Delivery Failed.
The notifications show different messages based on the status:
| Status | Message |
|---|---|
| Complete | Export package has successfully generated. |
| Error | Package failed to generate due to an error with the package. Please see log for issues. |
| Delivery Failed | Package successfully generated but failed to deliver. |
| Delivered | Package successfully generated and delivered |
Enablement & Configuration
This feature is enabled automatically, but an admin user must add users as subscribers for them to receive notifications.
Automated Checks
Use Case
The Automated Checks feature provides a way for data managers to automate parts of their cleaning processes, monitor it, and adjust as necessary, so that they can focus their efforts on discrepancies that truly need detailed review.
Description
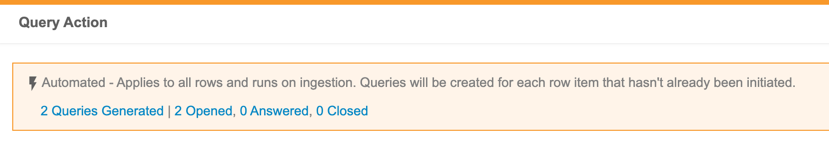
Users with the Create Listings permission can now create Checks. Within a Check, a user can define a query action on each Event Date or Item that exists on the check. CDB then opens a query against new records as they are captured by the Check. When a row is no longer captured by the Check (indicating that the issue has been resolved), CDB automatically closes the query.
This feature adds a new tab to CDB, Checks, which is accessible from the navigation drawer () and the Study menu. The review dashboard also includes a Checks widget.
Enablement & Configuration
This feature is enabled automatically, but CDB users must create checks for them to run.
Export Audit Logs
Use Case
This feature improves monitoring for exports in CDB. Users can access audit logs for events such as when a user downloaded a package, what listings was an export definition created with, and when listings were generated as part of what package.
Description
With this release, we added an Audit Logs page to Exports. This contains audit logs for Exports (Export Definitions), Export Packages, and Export Listings. The audit log records metrics around generation and delivery time. Users can choose the Log Type and Date Range of the audit event. Users can select a date range of 90 days at a time.
For Export Definitions, the Audit Log tracks the following events:
- Export definition created and what listings it was created with (the Audit Log shows the number, download the CSV for a list)
- Selected Listings modified and what listings were removed/added (the Audit Log shows the number, download the CSV for a list)
- Export definition deleted
- Export schedule created
- Export schedule modified
- Export schedule deleted
- Export delivery enabled
- Export delivery FTP connection modified
- Export delivery disabled
The Export Definitions log type includes the following columns:
- Log Type
- Timestamp (GMT)
- Export ID
- Export
- Type
- User
- Event
For Export Packages, the Audit Log tracks the following events:
- Package generation initiated
- Package downloaded
The Export Package log type includes the following columns:
- Log Type
- Timestamp (GMT)
- Export ID
- Export
- Type
- Package ID
- Package
- User
- Event
For Export Listings, the Audit Log tracks the following events:
- Export Listing generated in a package
The Export Listings log type includes the following columns:
- Log Type
- Timestamp (GMT)
- Export ID
- Export
- Type
- Package ID
- Package
- Export Listing ID
- Export Listing
- User
- Event
For export packages, users can also download a package log as a TXT file for an export package from Exports > Packages, which includes metrics around generation and delivery time. The file name format is “{StudyName}-{ExportDefinitionName}-Export_Log-{yyyy}-{mm}-{dd}T{HH}_{mm}_{ss}.csv”, with the datetime as the date and time the file was downloaded in GMT. This log includes the following information:
- Export Definition Type
- Listings
- Export Definition Created Date
- Export Definition Created By
- Schedule
- Scheduled By
- Delivery
- Package Format
- Package Processed By
- Package Process Start Time
- Package Process Completion Time
- Package Process Duration
- Delivery Start Time
- Delivery Completion Time
- Delivery Duration
- Size
- Oldest Date Applied
- Latest Date Applied
Enablement & Configuration
This feature is enabled automatically. Note that the audit log begins recording events at the time of release.
Additional Attributes to Sys_Forms Listing
Use Case
For raw-type exports, these additional attributes returned by Sys_Forms supplement contextual information about the data collected for the Study. These additional attributes also help customers in their transition to CDB.
Description
With this release, we added 16 additional attributes to the Sys_Forms system listing. These new attributes increase the total number of attributes returned by Sys_Forms from 17 to 33. This doesn’t impact any of the 17 attributes that were already returned by Sys_Forms.
These new attributes include:
- Site.PI
- Site.Country
- Site.Name
- Subject.Status
- EventGroup.SeqNbr
- Event.Date
- Event.Status
- Form.ILB
- Form.ILBReason
- Form.SDV
- Form.DMR
- Form.Frozen
- Form.Locked
- Form.Signed
- Form.LastModifiedDate
- Form.Version
- Form.ExternalID
Enablement & Configuration
This feature is automatically available, but for Export Definitions created prior to this release, users will need to regenerate the export package for it to include the new columns.
3rd Party Data Import Enhancements
Description
This feature includes the following enhancements:
- When a file contains a column without a header name, CDB generates the F-016 warning.
- If a third party data package doesn’t contain any data (CSV files only have a header row), CDB creates a blank Core Listing for the source.
Enablement & Configuration
This feature is automatically enabled.
CDB Usability Enhancements
Description
Users are now able to filter listings and checks with case insensitivity via contains, doesn’t not contain, starts with and ends with operators for string fields.
Enablement & Configuration
These changes apply automatically.
EDC Migrator
Features in this section are new features for Veeva EDC Migrator.
Source Data Exclusions (InForm)
Use Case
Sponsors can now define whether these deleted, but non-null, and “Not Applicable” records should be imported into CDMS as part of the migration process.
Description
Prior to this release, Migration Vault migrated all data in the package, even if that data was marked as Deleted or Not Applicable. With this feature, customers migrating Studies from InForm to CDMS can choose whether to exclude from the load any data marked as Deleted or Not Applicable, while still maintaining the necessary sequencing for Item Groups.
Deleted most often applies to Forms and Item Groups. These are most likely entered in error or created by the Site. By excluding these from the imported data, Migration Vault can migrated all data and maintain the Sequence Number of each Form or Item Group. For example, if Item Groups 1 through 5 are included in the package, but 2 is marked as Deleted, Migration Vault migrates 1 and 3 through 5, maintaining the Sequence Number of each.
Not Applicable is typically Events that were marked as Did Not Occur.
When this data is included in a migration, it creates additional overhead for sponsor teams to track that data and manually clear the records (via Intentionally Left Blank), in CDMS.
Enablement & Configuration
This functionality is automatically available, but a migration user must make changes to their YAML mapping files in order to use it.
Derived Columns
Use Case
Unlike Rave, the Names of Event Groups aren’t present in the source data for InForm Studies. With this feature, Migration Vault now supports multiple scenarios where a single Event has a distinct set of Forms for one Visit and a different set of Forms for another Visit. Previously, supporting this involved creating a single Event that contained both sets of Forms. This new method creates multiple, distinct Events with their own sets of Forms to mirror the way Studies are designed in CDMS.
Description
This feature provides the ability for migration users to map their source data to a Veeva Definition Name based on multiple CSV columns. Prior to this release, mapping was based on a single column.
Enablement & Configuration
This functionality is automatically available, but a migration user must make changes to their YAML mapping files in order to use it.
Source Stamping for Migrated Code Requests
Use Case
This additional property helps identify the origin of the code request and how it made its way into the system.
Description
With this release, Migration Vault stamps the Source Type for migrated code requests as “cdms_migration__v” (Migration Vault).
Enablement & Configuration
This change applies automatically to migration loads initiated after the release.
Migration Submits Forms with Derived Items Created by Repair
Description
With this release, if a Form contains a derived-type Item, and that derived Item is created by repair, Migration Vault submits the Form so that the item’s value can be derived.
Enablement & Configuration
This change applies automatically to migration loads initiated after the release.