What's New in 21R1
Pre-Release Date: March 29, 2021 | Release Date: April 9 & 16, 2021We are pleased to bring you Veeva Clinical Data in 21R1. Read about the new features below. You can find information on enabling new features in the 21R1 Feature Enablement Details. Information on developer features (REST API) is in the Developer Portal.
Clinical Data
Features in this section are changes that apply to all application areas of Veeva EDC and CDB.
SSO Authentication for eSignatures
Use Case
This feature provides the ability for Sponsors to manage users via an existing Single Sign-On provider and allows users to utilize their existing SSO credentials to perform tasks within Vault CDMS.
Description
With this release, Vault CDMS supports authenticating with a Single Sign-On (SSO) Provider while performing CDMS-specific activities that prompt users to authenticate after logging in. The following authentication prompts were updated to support SSO in addition to continuing authentication via Vault username and password:
- Signing at the Form level
- Signing at the Casebook level
- Accepting Study Closeout PDFs
- Randomization - Emergency Unmasking
- Randomization - Reveal Treatment
- Randomization - Redispense Kit/Device
- Randomization - Verify List
- Randomization - Invalidating Randomization
- Local Labs - Updating Normal Ranges in Use
When authenticating in one of the above flows as a user configured for SSO, the original dialogs will display without the username and password field followed by a dialog specific to their single sign on provider to sign in. Vault CDMS uses the existing SAML Profiles and Security Policies configurable at the Vault level.
Enablement & Configuration
This feature is available in all Vaults. SAML Profiles and Security Policies must be configured and assigned to Users in order to take advantage of this functionality within a Study.
Data Entry
Features in this section are changes to the Data Entry tab, a working area for investigators and clinical research coordinators to enter study execution data.
Data Entry General Enhancements
Use Case
These enhancements allow data entry users to more efficiently enter data by reducing the need to continually scroll the schedule and reapply expand all or collapse all.
Description
To improve the user experience, the Casebook Schedule panel of the Data Entry user interface no longer performs a full reload of the Schedule each time a Form or Event status updates. As users enter data, Vault updates the status icons, but the scroll position remains the same. This removes the need to scroll to the current Event each time an update is made.
Additionally, the Expand All Events/Collapse All Events setting is now sticky, meaning that once a user expands or collapses the schedule, Vault maintains their selection across pages, until they end the session. Now, users no longer need to reapply this setting every time they switch subjects or refresh the page.
Enablement & Configuration
These changes apply automatically in Studies using version 2 of the Data Entry UI.
Data Review
Features in this section are changes to the Review tab, a working area for clinical research associates and data managers, or to review functionality within the Data Entry tab.
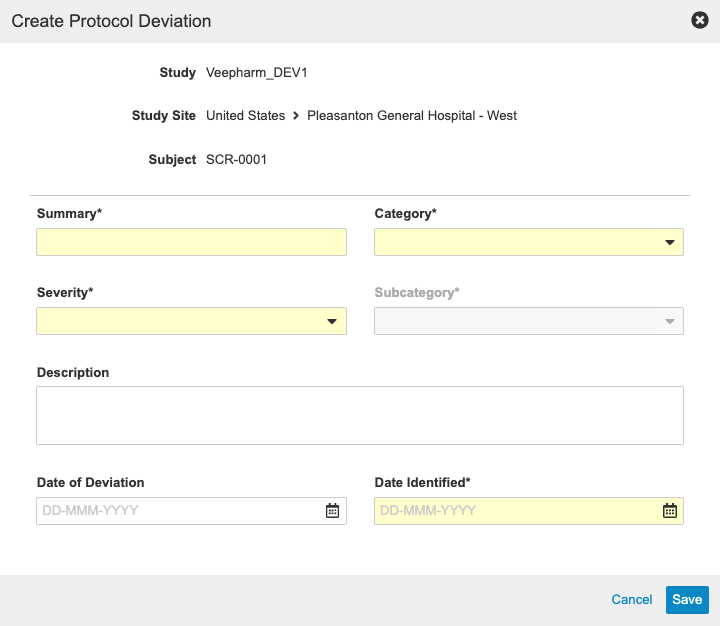
Protocol Deviations
Use Case
Sponsors now have the ability to create and review protocol deviations in the same system as they collect clinical data.
Description
With this release, sponsors have the ability to track Protocol Deviations for a Study from within Vault CDMS. Monitoring users can manually create Protocol Deviations from the Review tab, or the system may create a Protocol Deviation programmatically via a rule. Both manual and programmable protocol deviations are linked to a Subject, Event, Form, or Item, and each protocol deviation captures the following details:
- Summary
- Category
- Subcategory
- Severity
- Description
- Date of Deviation
- Date Identified
Once enabled in a study, study designers can configure their required Category, Subcategory, and Severity options for the Study within Studio. They can then also write Create Protocol Deviation rules to programmatically add protocol deviations based on user-entered data.
Configurations for protocol deviations are included in deployments and are documented in the SDS and version comparison.
Monitoring users can review Protocol Deviations from the new subtab, Review > Protocol Deviations. Users with the appropriate permissions can view Protocol Deviations for all Studies and Sites that they have access to from this area. Sponsors can update the status of a Protocol Deviation and provide resolutions during the review process from this area as well.
This feature adds the following new permissions:
- View Protocol Deviations: Ability to view Protocol Deviations from Review > Protocol Deviations (CDMS Clinical Research Associate, CDMS Data Manager, CDMS Lead Data Manager, and CDMS Auditor Read Only)
- Edit Protocol Deviations: Ability to edit Protocol Deviations (CDMS Data Manager, CDMS Lead Data Manager, and CDMS Clinical Research Associate)
- Create Protocol Deviations: Ability to create manual Protocol Deviations (CDMS Data Manager, CDMS Lead Data Manager, and CDMS Clinical Research Associate)
Enablement & Configuration
This feature may be enabled in all Studies by setting the Enable Protocol Deviations study setting to Yes. While manual Protocol Deviations are available in all Studies, programmable Protocol Deviations (those created using the Create Protocol Deviations rule action) are only available in Studies using version 2 of the expression grammar.
Veeva Coder
The following are new features for the Veeva Coder application, the Veeva Clinical Data solution for clinical coding.
Group Querying
Use Case
With this feature, coders can now stay in Group mode when coding and when sending queries to sites. When using Query Status as a criterion for grouping, Coders streamline by taking actions on a group based on the groups Verbatim, Coding Status, and Query Status.
Description
Coders can now open queries on a verbatim group while in Group mode. Opening a query on a group opens a query on each Code Request in the group.
As part of this feature, we added the ability to use Query Status as a criterion for grouping Code Requests. Other criteria include Verbatim, Coding Status, and Assigned Code (if present), as well as Indication, Route, or Seriousness, based on the form type and coding configuration. This is controlled by the Grouping Criteria for Verbatims Includes Query Status study setting.
Enablement & Configuration
The ability to open queries on all Code Requests in a group is automatically available. Including Query Status in the grouping criteria is auto-on for studies created after the 21R1 release (set to Yes in the Default Study Settings). For existing studies, a coder administrator can enable it with the Grouping Criteria for Verbatims Includes Query Status setting.
Coder Approval Workflow
Use Case
Organizations can have more experienced coders review coding decisions.
Description
When manually coded requests need review by a more experienced coder (coder manager), a study can enable the Approval Workflow. When enabled, all manually coded Code Requests must be approved. After a coder assigns the code, the Code Request moves into the Pending Approval status. Then, a manager can either approve or reject it. Approval moves the request into the Coded status. If the manager rejects the assigned code, they can provide a comment to explain their reasoning. Vault posts this comment as a Note on the Code Request.
The Approve and Reject actions can only be performed while in Group mode. When approving or rejecting, the manager can apply the same action to the matching Synonym in the Synonym List. Doing so marks the matching Synonym as approved or rejected.
We added the Pending Approval column to the Summary page to show the number of Code Requests in the Pending Approval status for a form, as well as the Coded and Autocoded columns. We removed the Pending Review and Approved columns.
As part of this feature, users can now change which columns display in both Group and List modes. This is useful for managers if they want to see additional attributes in the Code Request listing, such as Last Coded By or Last Coded Date.
Enablement & Configuration
This feature must be enabled by a coder administrator from Tools > Coder Tools > Study Settings. To enable this feature, set Enable Approval Workflow to Yes. The Approval Workflow uses Coded as the final, approved status so that studies using this feature and studies not using this feature use the same final status for Code Requests.
Select All Forms for Batch Upversioning
Use Case
This change allows easier and faster batch upversioning, especially for updates to large, live Studies.
Description
With this release, users may now select more than twenty (20) forms to batch upversion using the new “Select All Forms on All Pages” option, instead of upversioning Forms in groups of twenty.
Enablement & Configuration
This feature is automatically enabled in all vaults with the Vault Coder application.
Preferred Base for Extracts
Use Case
Data teams no longer need to lookup this data, it will be available directly in listings and extracts.
Description
Coder Administrators can choose to store the Preferred Base in the database for all assigned codes. When enabled, Vault includes the Preferred Base in all listings and extracts, including Core Listings, the Data and Definition Extract, and the Code Request Extract.
Enablement & Configuration
This feature must be enabled by a coder administrator from Tools > Coder Tools > Study Settings. To enable this feature, set Store Preferred Base to Yes.
Study Design & Configuration
Features in this area apply to Studio, the study design and configuration area for Veeva EDC.
Library Collections
Use Case
Library collections provide a separate and secure method to manage study standards.
Description
This release introduces a new Library sub-tab under Studio (the current Studio area may be accessed from Studio > Studies). From the Library, librarians can create a new type of Study called a Collection. A collection allows an organization to manage a set of standards isolated from other Studies and only editable by a user with the CDMS Librarian role. Study designers can be given view access to a Collection, but they can’t edit them.
In the New Study dialog, study designers can now copy from a library Collection or from an existing Study. When copying from the library, Vault doesn’t include any configuration for Randomization.
Collections use deployment for versioning, and annotated PDFs, the SDS, and the comparison report are available for Collections as they are for regular Studies.
Enablement & Configuration
This feature is automatically enabled in all vaults.
Rules Enhancements
Use Case
This feature removes the need to create multiple rules when multiple Event Groups are added to the schedule based on the same logic.
Description
With this release, we made the following enhancements to rules:
- For Add Event Group rules, Study designers may select multiple Event Groups to add to the study schedule, instead of creating a rule for each Event Group with the same expression.
- Date comparison rules may now specify that two dates or datetimes are equal.
Enablement & Configuration
These enhancements are available automatically in studies using version 2 of the expression grammar.
Library Report
Use Case
This feature allows librarian’s to understand how study designers are leveraging the library and provides insight into deviations from study standards. This knowledge helps guide further investment into the library or can help tune study design processes.
Description
Librarians and study designers can create a vault-level report that shows definitions, their keys, where they originated,and which versions they changed in. The goal of this report is to allow librarians to identify what objects were sourced from the library and if they were changed since they were copied from the library. The report uses the Cross Vault Unique ID to identify the Source Version and Changed In Version. Librarians can run the report via the Create Library Report action from the Actions menu in a library Collection.
The report includes Forms, Item Groups, Items, Codelists, Units, and Rules. Vault creates records when the definition is new to a Study, either by copying it from another study or creating it manually. Vault updates records when they are changed in that version. If a definition was created as the result of a new version, but it wasn’t changed, it reflects the new version.
In this release, the report doesn’t roll up changes to the form level, so if further insight is required to understand a deviation, a library may need to leverage a study’s SDS to identify all impacted forms.
Vault does not create lineage records for existing Studies. These records may only be created from the release point forward. Organizations can work with Veeva Services to seed library lineage records for existing studies if required, but this is only possible when the existing Study is converted to a library Collection. For these seeded records, Vault creates a change for each version of that converted study. During seeding, users will not be able to create or edit definitions in the collection.
Enablement & Configuration
This feature is automatically available for library Collections. If required, organizations can work with Veeva Services to convert an existing study to a Collection to leverage this report.
Access Data from Linked Forms with Rules
Use Case
This feature improves the variety of rules that study designers can write and enhances the form linking feature.
Description
Study designers can now include identifiers from linked forms in expressions to take full advantage of the form linking functionality. Rules containing at least one link identifier are evaluated when two forms are linked, as well as when each of the linked forms is resubmitted. When a link identifier is added to an expression, there are limitations on which other identifiers can be added.
Enablement & Configuration
This feature is automatically available for all Studies using version 2 of the expression grammar. Study designers can begin writing rules that access linked forms after the release.
Study Administration
Features in this section apply to System Tools or EDC Tools, a study-level administration area for Veeva EDC.
Study Data Extracts Job
Use Case
Users have access to the SAS version of all Clinical Data in a form, including system data without using utilities.
Description
This feature will allow Data Managers to have access to all Clinical Data in a form including system data (e.g., operational, queries, review, etc.) in EDC tools. Users will have the option to download the data as a SAS or CSV file. In addition, all DateTime fields will be shown in Site Timezone, UTC Timezone, and User Timezone in the Data Extracts, Core Listings, and Data and Definition Export jobs.
Enablement & Configuration
This feature is available automatically, with no additional configuration required.
Job Enhancements
Use Case
Lead data managers can now cancel long-running jobs.
Description
With this release, we made the following enhancements for job management:
- Users can cancel an in progress Core Listing, Retrospective Amendment, Labs Mass Updates, or Data and Definition Export job.
- Users can refresh the Job History without refreshing the entire page.
- Any output or job log files are now available for download for up to 6 months. The previous window was 15 days. This applies to any jobs run after the release. Any earlier jobs remain on the 15-day window.
Enablement & Configuration
These changes apply automatically, with no additional configuration required.
EDC Tools General Enhancements
Use Case
These enhancements improve usability in EDC Tools.
Description
With this release, users can choose the status – “In Progress” or “Submitted” – for changed forms when creating a new Retrospective Amendment in EDC Tools.
Enablement & Configuration
These changes apply automatically, with no additional configuration required.
Additional Columns for DateTime Items in Extracts
Description
The Core Listings, Data and Definition Export, and Study Data Extracts job outputs now show the entered datetime in the Site’s timezone, UTC timezone, and the timezone of the user who ran the job, all in ISO format yyyy-MM-dd HH:mm:ss.
Enablement & Configuration
This change applies automatically with no configuration required.
Randomization
Features in this section are new features for the Randomization module of Veeva EDC.
Export Randomization Lists
Use Case
This feature allows the user to export only relevant data using filters.
Description
Randomization lists can now be exported. The export will be based on the filtered view and the visible columns displayed.
Enablement & Configuration
This feature is automatically available in Studies using Randomization.
Emergency Unmasking Report
Use Case
Organizations can easily track when subjects are unmasked and by which users.
Description
The Emergency Unmasking Report lists all instances where a user performed an unmasking on a subject in a masked study. The report shows the user who performed the unmasking and the date and time the masking was performed.
Enablement & Configuration
This report is automatically available in Studies using Randomization.
Search & Filter for Randomization Lists
Description
This enhancement allows users to filter their randomization lists and devices and kits by Subject Status, Site, Strata Group, Randomization Status, and more as well as search for individual list items.
Enablement & Configuration
This feature is enabled automatically in Studies using Randomization.
Labs
Features in this section are new features for the Labs module of Veeva EDC.
Show Number of Pending Lab Locations
Use Case
This number indicator lets the user know if there are lab locations waiting for their approval without having to manually check the tab.
Description
The Pending Lab Locations tab now has a number indicator to show how many Lab Locations are pending for approval.
Enablement & Configuration
This feature is enabled automatically in Studies using Labs.
Recalculate Age
Use Case
A subject’s age must be recalculated if the user updates the birth date or birth year after the Lab collection date and time has been entered. The “Recalculate Age” feature allows the user to recalculate a subject’s age at the click of a button without the user needing to clear the Lab Collection date and time to update to the correct age.
Description
Users can recalculate a subject’s age based upon the Lab Collection Date and the Birth Date or Birth Year item.
Enablement & Configuration
This feature is automatically available in Studies using Labs.
Role Management & Security
Features in this section are enhancements to the System Tools > Role Management and System Tools > Users areas, as well as changes to standard Study Roles, security, and access control in Veeva Clinical Data.
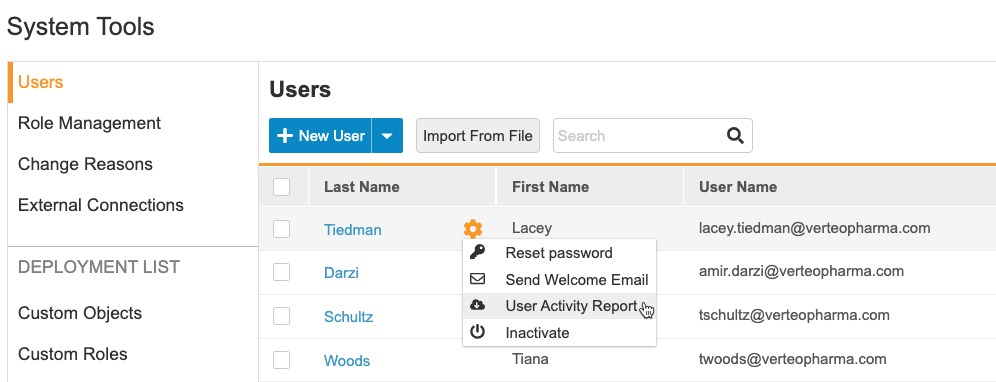
Vault-level User Management
Use Case
User administrators can now change a user account’s status and reset a user’s password without Vault Owner level access.
Description
User management is now at the vault level. User administrators may create user accounts and assign study access to any studies in that vault from one location (Tools > System Tools > Users). They can also reset a user’s password, resend a welcome email, and active or inactivate users. The User Activity Report is also available from this page. This feature moves all user management activities from EDC Tools > Users to Tools > System Tools > Users.
Enablement & Configuration
This feature is enabled automatically in all vaults.
Connections & Integrations
Features in this section are new connections or integrations with Veeva Clinical Data or enhancements to existing ones.
Safety Link
Use Case
This integration automates the reporting and follow ups for serious adverse events and related forms between Vault CDMS and a Safety System of choice, in compliance with FDA requirements for timely reporting of SAEs.
Description
Safety Link is an integration between Vault CDMS and Vault Safety or between Vault CDMS and any third party Safety System via the CDMS AS2 Gateway. Enabling this integration automates the reporting of all serious adverse events (SAEs) to the chosen safety system. Organizations can configure a study to also send related (via form linking) case information, including concomitant medications, medical histories, and related adverse events, alongside the SAE, and optionally demographics items or an In Case of Death form, when that data is available. Any time a site completes or updates the SAE form, or any related forms, CDMS automatically generates a first-send or follow-up and sends it to the safety system.
The automated data transfer uses E2B in the R3 format. This provides a scalable way to send all required and complimentary case information that any safety system can parse and ingest accurately.
Administrators can configure this integration by mapping study design Items to the E2B R3 elements and setting the timing of the first-send and the frequency of any follow-ups. For third-party safety systems, they can provide other attributes, such as a URL and Certificate, to use with the integration.
Administrators can configure the integration directly in the production environment, or configure it in a UAT environment for testing before exporting it and importing it into the production environment. They can also export the configuration for use in other Studies and Vaults.
Safety Link supports automated nullifications via E2B in R3 format. This is generated if the SAE Term is changed to non-serious or the form is reset. Safety Link accepts Acknowledgements from the safety system to confirm that the data was received, parsed correctly, and confirm the case status, message status, and provide error details, if any. All this data is readily available in the Safety Cases and Safety Message reports to confirm the integration status and health.
The reports also support reconciliation workflows. The reports include CDMS Case ID, Case Status, Site, Subject, Form Reference Number. This can be combined with a SAE Listings report which includes the SAE Term, SAE Related Item Values, Coding values and metadata, Site, Subject, and Form Reference Number. Compare this combined report side-by-side with the reports available in the safety system.
Enablement & Configuration
Contact Veeva Support to enable this feature in your vault. Your organization must have a safety system, and if your safety system is not Vault Safety, then your safety system must also have an AS2 Gateway to manage file transfers. This is only available to new Studies.
Procedure Notifications for Vault CTMS
Use Case
EDC can now provide CTMS with payment related information extending platform capabilities and adding value to customers.
Description
EDC can now transmit mapped submitted Procedures to Vault CTMS when this integration is enabled. The procedures are used for payments in CTMS. Integration managers must map Procedures on a study-by-study basis in EDC Tools. EDC creates a Procedure record when a procedure-mapped Form is submitted by a data entry user. Then, EDC sends a message, via Spark, to CTMS to inform the system that a Procedure was created. In addition to form submission, the integration is able to update the status of deleted and reset Forms. The Integration Manager can map form deletion and reset to the appropriate statuses in EDC Tools.
This feature introduces the Integration Configurations area in EDC Tools. From the Integration Configurations area, lead data managers can create configuration records required for integrations. Integration Configurations supports platform integrations via Spark from custom objects populated by lead data managers on this page. Configuration is available for the following form-level actions: Submitted, Delete/Reset, Linked, and Unlinked.
Enablement & Configuration
While this integration is automatically enabled, it must be configured for a Study in EDC Tools. An organization must also have a CTMS application vault.
Clinical DataBase (CDB) & EDC Clinical Reporting
The following are new features for the Veeva CDB application, EDC Clinical Reporting (the Veeva Clinical Data solution for data cleaning and reporting), or both.
Availability: Clinical DataBase (CDB) is only available to CDB license holders. Contact your Veeva Services representative for details.
Enhanced Security for CDB
Use Case
These new permissions offer more granular control over which user roles can perform which functions in CDB.
Description
With this release, we added several CDB-specific functional permissions, as well as a new standard Study Role, CDB Data Provider. The CDB Data Provider role is intended for vendor users. The CDB Data Provider has the Workbench Tab, Restricted Data Access, and API Access permissions. In support of future enhancements, we added the Admin page to CDB.
Organizations should plan to update their custom Study Roles upon the release of this feature to assign these new permissions.
| Permission | Description | Assigned To |
|---|---|---|
| Edit CQL | Ability to edit the CQL statement for a listing in the CQL Editor | CDMS Data Manager, CDMS Lead Data Manager |
| Modify Listing | Ability to edit the CQL statement and properties of private listings. *Includes public and export listings when combined with the Public Access permission | CDMS Data Manager, CDMS Lead Data Manager |
| Create Listing | Ability to create private listings *Includes public and export listings when combined with the Public Access permission |
CDMS Data Manager, CDMS Lead Data Manager |
| Delete Listing | Ability to delete a public listing | This permission isn't assigned to any standard roles. |
| Generate CSV | Ability to generate a CSV for a listing | CDMS Data Manager, CDMS Lead Data Manager |
| Public Access | Ability to create or modify a public listing, when combined with the Create Listing and Modify Listing permissions | CDMS Data Manager, CDMS Lead Data Manager |
| View Listings | Ability to access the Listings page | CDMS Data Manager, CDMS Lead Data Manager |
| View Queries | Ability to access the Queries page | CDMS Data Manager, CDMS Lead Data Manager |
| View Export | Ability to access the Export page | CDMS Data Manager, CDMS Lead Data Manager |
| Create Export Definition | Ability to create and copy Export Definitions | CDMS Data Manager, CDMS Lead Data Manager |
| Generate Export Package | Ability to generate a CSV or SAS export package | CDMS Data Manager, CDMS Lead Data Manager |
| Delete Export Definition | Ability to delete an Export Definition | This permission isn't assigned to any standard roles. |
| View Export Packages | Ability to access Export > Packages to view generated export packages | CDMS Data Manager, CDMS Lead Data Manager |
| View Import | Ability to access the Import page | CDMS Data Manager, CDMS Lead Data Manager |
| Download Import Package | Ability to download import packages | CDMS Data Manager, CDMS Lead Data Manager |
| View Admin | Ability to access the Admin page | CDMS User Administrator |
Enablement & Configuration
These permissions changes apply automatically in vaults that contain the CDB application.
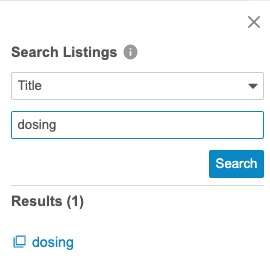
Listings UI Improvements
Use Case
With more and more custom listings being generated for a given study, it is critical for data managers to be able to quickly navigate to listings of interest.
Description
Workbench users can now sort, filter, and search within the Listings page’s four tabs to more easily locate listings of interest. Users can also use the new Show CQL action to view the CQL statement for a listing without having to open the listing.
Enablement & Configuration
This feature is available automatically.
Union Behavior Changes
Description
With this release, the default behavior for UNION is the same as UNION ALL. This returns all rows to all results from queries in the union. We then added the UNION DISTINCT function, which returns the results from queries in the union with duplicates removed. UNION DISTINCT only removes duplicate rows when the duplication is a 100% match.
Enablement & Configuration
These changes apply automatically.