Importing & Exporting a Casebook Definition (ODM)
You can export and import your Casebook Definition as an ODM XML document.
Legacy Feature: The ability to import and export a study’s design as an ODM XML document is a legacy feature. As of the 19R3 (December 2019) release, it is no longer actively maintained.
Prerequisites
Users with the CDMS Study Designer study role can perform the actions described below by default. If your vault uses custom Study Roles, you must have the following permissions:
| Type | Permission Label | Controls |
|---|---|---|
| Standard Tab | Studio Tab | Ability to access the Studio tab |
| Functional Permission | Design Study | Ability to create study design definitions and a study schedule from Studio |
If your Study contains restricted data, you must have the Restricted Data Access permission to view it.
Learn more about Study Roles.
Automated Deployment Studies
For post-19R3 Studies you can use deployments in EDC Tools to move your study design from environment to environment. You can only use import when creating the first casebook version. Once you’ve deployed the first casebook version of your study, import is no longer available. You can still export your Casebook Definition, regardless of casebook version.
About the ODM XML File
Vault uses a Veeva Extended ODM XML file for importing and exporting Casebook Definitions. Vault includes the following in the ODM XML file, depending on your configuration and your selections when exporting:
- All design definition records, including any configuration performed in the Properties panel
- Study schedule and layout relationships between design definitions
- Coder Study Settings, including the assigned dictionary release, Synonym List, Stop List, and Therapeutic Area for the study (if selected during export)
- Rules (if selected during export)
- Views (if selected during export and this feature is enabled in your vault)
The following configurations are not included in the ODM XML file:
- Review Plans
- Assessments
- Form Links
- Settings on the Study Configuration object record
- Any configuration performed in EDC Tools
- Progressive Display configuration
- Protocol Deviation configuration (Categories, Subcategories, and Severities)
- Rules with the following action types:
- Send Email
- Create Protocol Deviation
- Comparison Rules
- Casebook Variables
You must perform these configurations in each Study.
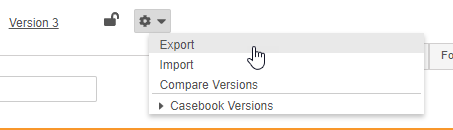
How to Export a Casebook Definition
To export a Casebook Definition:
- In Studio, navigate to the Study that you want to export.
- Optional: You can choose a version other than the most recent version. From the Actions menu, click to expand Casebook Versions and choose which version to export.
-
Select the checkboxes of the components you want to include in the export.
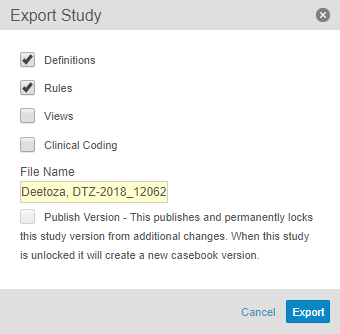
- Optional: Enter a File Name. By default, Vault names the file with the Study Number and the export date.
- Click Export. When the export is complete, Vault sends an email notification, which includes a link to download the ODM XML file and a summary CSV file, which indicates any Item Definitions that Vault skipped exporting due to errors.
Preparing the File for Import
To import a Casebook Definition, the OID for the Study in the XML file must match the OID of the target Study. In the Studio UI, you specify an OID in the External ID field.
Before importing a Casebook Definition, update the XML file and replace the OID with the OID of the target Study. The OID is listed twice in the beginning of the XML file, as an attribute on the AdminData and Study tags.
<?xml version="1.0" encoding="UTF-8"?>
<ODM xmlns="http://www.cdisc.org/ns/odm/v1.3" xmlns:veeva="http://www.veeva.com/ns/veevaedc/v1.0" FileType="Snapshot" FileOID="OOZ000000000101" CreationDateTime="3/5/2020 11:17:44 AM PST" veeva:VeevaODMVersion="1.0.7">
<AdminData StudyOID="S.CHOLECAP">
<veeva:StudyConfiguration veeva:ExpressionEngineVersion="2" veeva:ReviewPlanOverride="true" />
</AdminData>
<Study OID="S.CHOLECAP">
...
</Study>
</ODM>
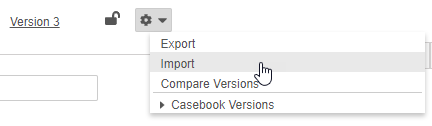
How to Import a Casebook Definition
To import a Casebook Definition:
- In Studio, navigate to the Study for which you want to import a Casebook Definition.
-
In the Import Study dialog, browse for and select your Casebook Definition ODM XML file.
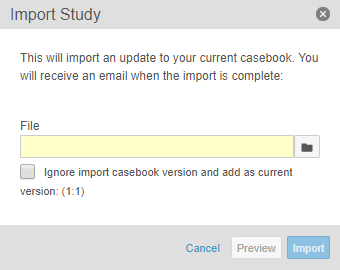
- Optional: Select the Ignore import casebook version and add as current version checkbox to import the Casebook Definition into the current version and publish status. This is only available for the first casebook version for a Study.
- Optional: Click Preview. Vault imports a preview version of the Casebook Definition. Note that you will need to perform steps 1 through 3 a second time to fully import the Casebook Definition.
- Click Import. When the import is complete, Vault sends you an email notification.
If your study uses the automated deployment model, Vault imports as the first casebook version automatically because you can only import for the initial version of your study.