Tips & Best Practices for the Study Schedule
This page lists various tips and best practices to help you as you build your study’s schedule.
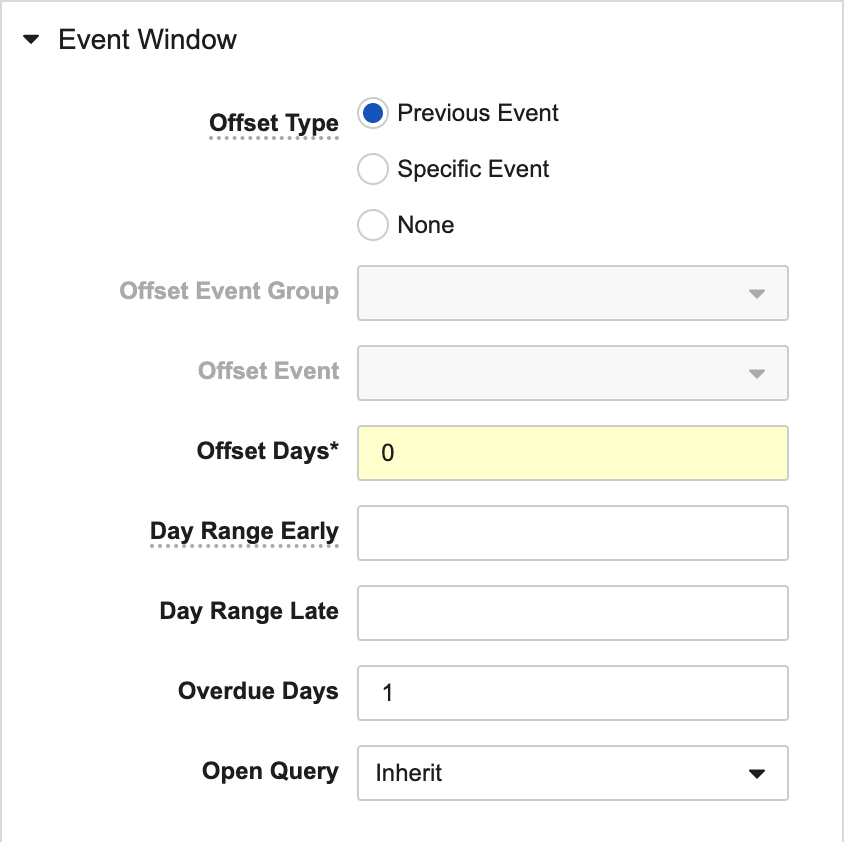
Don’t Forget to Add Event Windows
Setting the Event Windows properties (Day Range Early, Day Range Late, Offset Days, Offset Event) for your Events will allow Vault to show the Planned Date ranges for each Event in the Data Entry tab. If the Open Query property is set to Yes (or Inherit for Studies where Open Query on Out of Window Event Dates is set to Yes), Vault will open a query on the Event Date if the date is outside the planned range. This reduces the number of query rules required and supports reporting on overdue events.
Be sure to confirm that the Open Query on Out of Range Event Dates setting is set to Yes in your study’s settings.
Plan to review the window settings with your sponsor. You can use the Study Design Specification (SDS) during this review, which lists event window details in the Schedule - Tree worksheet.
You can add or adjust event windows as needed with protocol amendments.
For some events, there might not be a typical allowance of a number of (+/-) days. For example, if one visit is expected to occur one day after another visit, such as Visit 1 being one day after the Baseline Visit. In this example, you would use the do the following for the Visit 1 event window:
- Enter “1” for Offset Days and Overdue Days
- Select the Baseline Visit as the Offset Event.
- Leave the Day Range Early and Day Range Late properties blank or set them to “0”.
Set the Overdue Days
Check with your sponsor if there are expectations for timely data entry and set the Overdue Days property accordingly. An overdue form occurs when the Form is incomplete, and the number of days between the current date and the Event Date is greater than the specified number of Overdue Days. In the Data Entry and Review tabs, Vault notifies users of overdue forms by changing the form’s status to Overdue, which displays the red Overdue status icon. Vault also shows an Overdue Forms count in the Review tab for data managers and CRAs, as well as in the Standard Template: Overdue Form Entry per Event report and any custom reports that show overdue forms.
Overdue status icon:
Best Practice: Select the Future Date checkbox on all Events. When this is selected, Vault opens a query on any Event Date entered that is in the future at the time of entry.
Repeating Event Groups (Cycles)
Use repeating Event Groups for cycles, periods, or phases where the visits repeat. Even if there are different Forms in the Events, or different sets of Events per cycle, still plan to use a repeating Event Group. You can create rules to add the Forms or Events in certain cycles by referencing the event group’s Sequence Number in the rule expression.
Rules can also evaluate Cohort within the expression logic to easily add the appropriate Event Groups or Events as needed, reducing the number of Events and effort required to set up the study schedule.
Consider monotherapies versus combination therapies as an example. Depending on how much the schedules differ, you may still want to consider separate Event Groups if it makes sense to do so.
Name and Label Recommendations
Keep the following recommendations in mind for Names and Labels of event groups:
- Keep the Name and Label generic, such as eg_CYCLE/Cycle or eg_TREATMENT/Treatment.
- Subsequent Event Groups can use the same Label, if needed, as Vault only requires the Name to be unique.
- Don’t use numbers in the Name or Label, as these don’t represent the specific cycle. It can still be appropriate to include numbers in the names of non-repeating Event Groups as these represent a static and specific group of events.
Best Practice: When adding a repeating Event Group, you can use the Prefix Label in Related Events property to append a label to each Event label, such as Cycle # - Event Label. Keep the Name and Label for Event Groups generic, such as Cycle (eg_CYCLE) or Treatment (eg_TREATMENT).
See Naming Conventions for further recommendations.
Common Forms Event
The Common Forms Event is a log-type Event that contains both repeating Forms, such as Adverse Event or Concomitant Medication, and non-repeating forms such as Treatment Discontinuation, Death Data, and End of Study. These Forms aren’t associated with any scheduled study Events, and so they aren’t associated with an Event Date. Depending on your protocol, you may want your common forms to be included in your casebook immediately. To do so, add the log-type Event you created into the same Event Group as your Screening Event. Because Vault adds the first Event Group in the schedule to the Casebook upon creation, instead of by a rule, your Common Forms will display in the Casebook immediately.
Use Did Not Occur Instead of DOV Forms
Don’t use Date of Visit (DOV) forms, as these forms aren’t needed in Veeva CDMS. Instead, site users can mark Events as Did Not Occur to indicate when a visit doesn’t happen and select the reason. Vault allows for custom reasons, which study designers can configure in System Tools > Change Reasons.
Vault includes Event Dates in Study Data Extracts, both in the dataset for each individual Form and the overall event dataset (SYS_EVT).
Building the Schedule Dynamically
We recommend that you add Events to the schedule dynamically. You can add all Events in an Event Group to the schedule at once when you use a single Add Event Group rule to add the Event Group. You can also use Add Event rules to add individual Events to the schedule, even after the Event Group is on the schedule. Events can be included in an Event Group and rolled out together when a single rule is used to add the whole Event Group. It is recommended to roll out the events progressively, by evaluating the previous event’s Event Date to check that the date is entered or the Did Not Occur reason is equal to the applicable values such as “Subject missed event”. See best practices for Add Event rules.
End of Study & Early Termination
There are a few methods to handle the End of Treatment or Early Termination events:
- Create the Event with the Unscheduled event type. This allows site users to add it if or when they need it.
- Add the Forms for end of treatment or early termination to your log-type Event containing your other Common Forms and use rules to add the associated Early Term, End of Study, or Follow-up events.
- Create a separate Event Group for early termination and add it with a rule that checks for an Event marked as Did Not Occur with the “Early termination by subject” reason. Then, create additional rules to add treatment events based on presence of an Event Date for the previous event or the reason for Did Not Occur being “Subject missed event” (or any other custom Change Reasons for Did Not Occur). In this design, site users won’t need to complete all the Events before getting to the Early Termination or final visit.