Clinical Data Transformation & Extraction
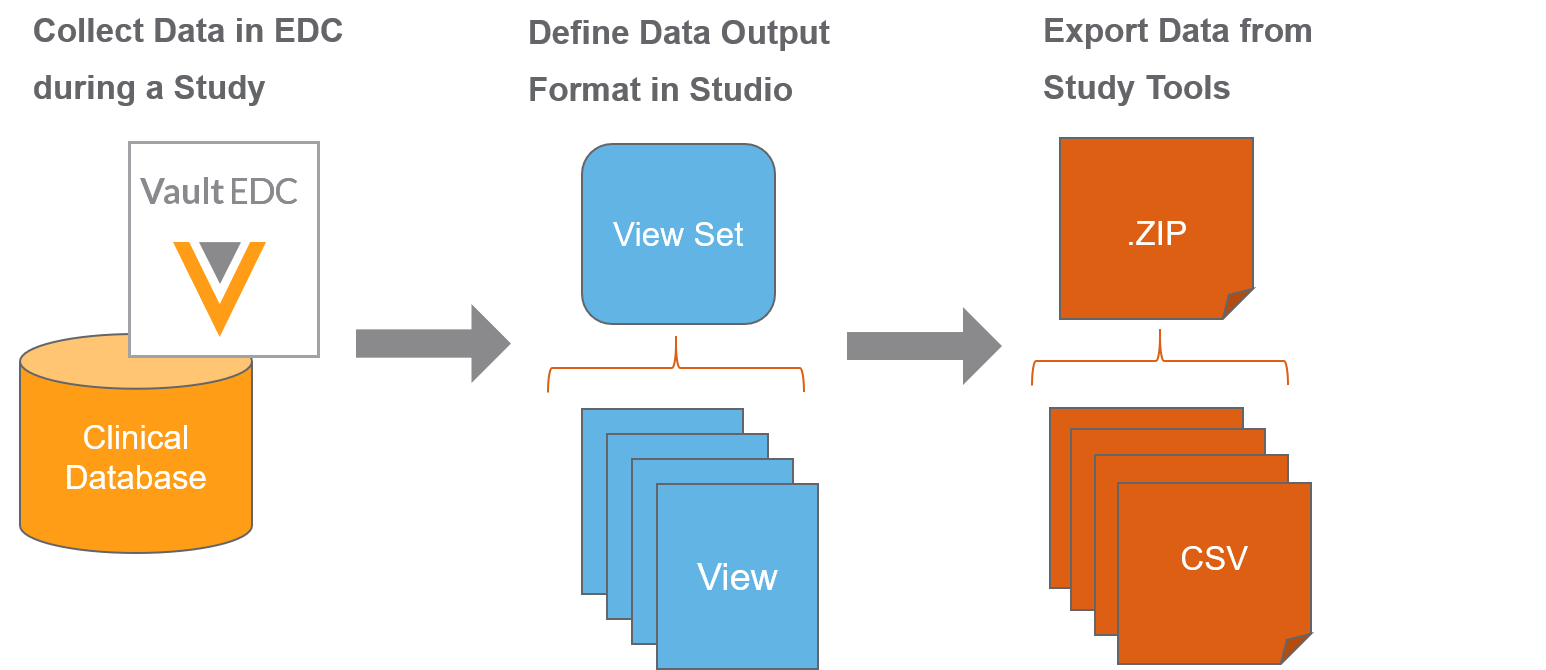
Vault EDC supports not only the collection of clinical data, but also the transformation and extraction of that data into a usable format. Vault EDC uses the view functionality set to support this use case.
The View Sets tab may not be enabled in your vault. Contact your Veeva Services representative for details.
Configuration to Output
Vault EDC makes planning and transforming data for export and analysis easy with Studio’s View Editor and the Data Export job in Tools >EDC Tools. You’ll first create a set of View Definitions in Studio. Then, you, or another user, will initiate an ad hoc or scheduled, repeating Data Export job to export execution data in your configured format. Lastly, once that job executes, a bio-statistician or clinical data manager can review the output CSV files, as well as import them into external tools for additional transformation and analysis.
Creating a View Definition
In Studio, you can create a set of View Definitions. Each of these View Definitions defines what data will display in the eventual CSV data output. You can create columns and rows, identify data points for export, and derive values in Studio’s View Editor.
Initiating a Job
Once you finish creating your View Definitions, you or an administrator can initiate a job to export data based on those View Definitions from EDC Tools > Jobs. Users with permission to access the Review tab can also export data based on those View Definitions from Review > Study Jobs. Vault EDC creates a CSV for each View Definition in a View Set Definition, and exports those CSVs in a single ZIP file. You can configure this job to run once or on a recurring, scheduled basis.
ZIP & CSV Output
Once the Vault completes the Data Export job, you can extract the CSV files from the output ZIP file. You can then review the data within those CSV files or import it into an external tool for additional transformation and analysis.
Views Data Model
Much like study design, Vault EDC uses definition and execution objects for view configuration. Definition object records are created within Studio. Vault creates execution object records for views automatically when a EDC Tools user initiates a Data Export job. You can easily identify definition-type object records as those records you create in Studio or any records in an object with “Definition” appended to the name.
Studio users can create View Set Definitions and View Definitions using Studio’s Views Editor, in Studio > View Sets. Each View Set Definition contains one more View Definitions. That View Definition contains the columns (Column Definitions), bindings (Column Binding Definitions), and rows (Column Row Definitions) Vault uses to collect data into a view.
Each time a EDC Tools user initiates a job to export data based on a View Set Definition, Vault creates a new records to represent the views resulting from the export. For each Data Export job, Vault exports a View Set Definition as a ZIP file, containing a CSV file for each of the View Definitions in the set.
Features
View Set Management
From Studio > View Sets, you can view, create, and edit your View Set Definition records from a single page, without leaving the context of Studio.
Views Editor
Studio includes an enhanced views editor, where you can both create and edit views, as well as copy views and perform additional configuration.
Multiple Column Types
Vault EDC supports three types of columns for views: static, direct, and derived.
- Static columns populate a single, entered value for every row in the view.
- Direct columns populate each row with the value of a specified object field.
- Derived columns allow you to create a formula, the result of which Vault uses to populate the row.
Support for Required Data
Clinical programmers are now able to set a Column Binding Definition to Display Null Values. If at least one of the Column Binding Definitions is set to Display Null Values for a column mapping row (shared Order value) is matched by instance data, then the entire row must be exported. For example, a View Definition has columns defined as: Subject, Gender, Cohort, Height, Weight, Pulse. A data manager may only need a Subject row to populate in the data export when there are values for Height, Weight, and Pulse. Subject, Gender, and Cohort are only contextual data and are not needed if there is no data for the other three columns. A clinical programmer can mark the Height, Weight, and Pulse columns as Display Null Values, and then when a data manager uses that View Definition for data export, the resulting View will only include rows for which there are values for the required columns, Height, Weight, and Pulse.
SAS-Enabled Views
Clinical programmers can configure their View Definitions so that they may be used to export data in SAS-format. SAS-enabled views are configured as regular, CSV-format views in the Studio Views Editor, but also require a small amount of additional configuration in the SAS Format Panel. Once configured, EDC Tools users can initiate a Data Export job, exporting a ZIP file containing a SAS file for each view within the set. You can then open and interact with these files in your chosen SAS viewer.
Study Data Extraction
Study administrators and clinical programmers can now auto-generate a View Set Definition containing a View Definition for each Form Definition in the Study. Each column on a form’s view maps to an Item Definition referenced in the study’s Casebook Definition. The main study extract View Set includes a full clinical data dump containing all data points collected within the Study.
Views for Connecting with Other Applications
Clinical programmers can now define subject milestone exports in Vault EDC that update subject information in an associated CTMS vault for use with operational reporting. The export from Vault EDC contains the following subject information: Study, Study Country, Study Site, Subject ID, Subject Status, Screened Date, Screen Failed Date, Randomized Date, Enrolled Date, Withdrawn Date, End of Treatment Date, End of Study Date. Users with access to both the EDC and CTMS vaults can open a subject’s Casebook in the EDC vault from their CTMS vault.
In addition to connecting with Veeva CTMS, you can create CSVs with views for integrating with various other external tools. Contact Veeva Services for information about custom integrations.
Uses
- Extracting all study execution data into a manageable CSV or Excel format
- Exporting data for analysis in external tools
- Connecting with Veeva CTMS to export Subject milestone data for operational reporting
Example: Subject Informed Consent Listing
You can use views to easily generate a listing of all Subjects in your Study and their informed consent status. You could create a View Definition with columns to show the following:
- Study Number: Direct mapping to the Name (
name__v) field on the Study (study__v) object. - Subject ID: Direct mapping to the Subject ID (
subject_id__v) field on the Subject (subject__v) object. - (Item) Age at Screening: Derived mapping, calculating the subject’s Age as the difference between their date of birth and the screening’s Event Date.
- (Item) Able to Understand and Sign an Informed Consent: Direct mapping to the Value (
value__v) of the Item (item__v) with the identifier{Inclusion Criteria/Able to Understand and Sign an Informed Consent}. - (Item) Informed Consent Signed: Direct mapping to the Value (
value__v) of the Item (item__v) with the identifier{Informed Consent/Informed Consent Signed}.
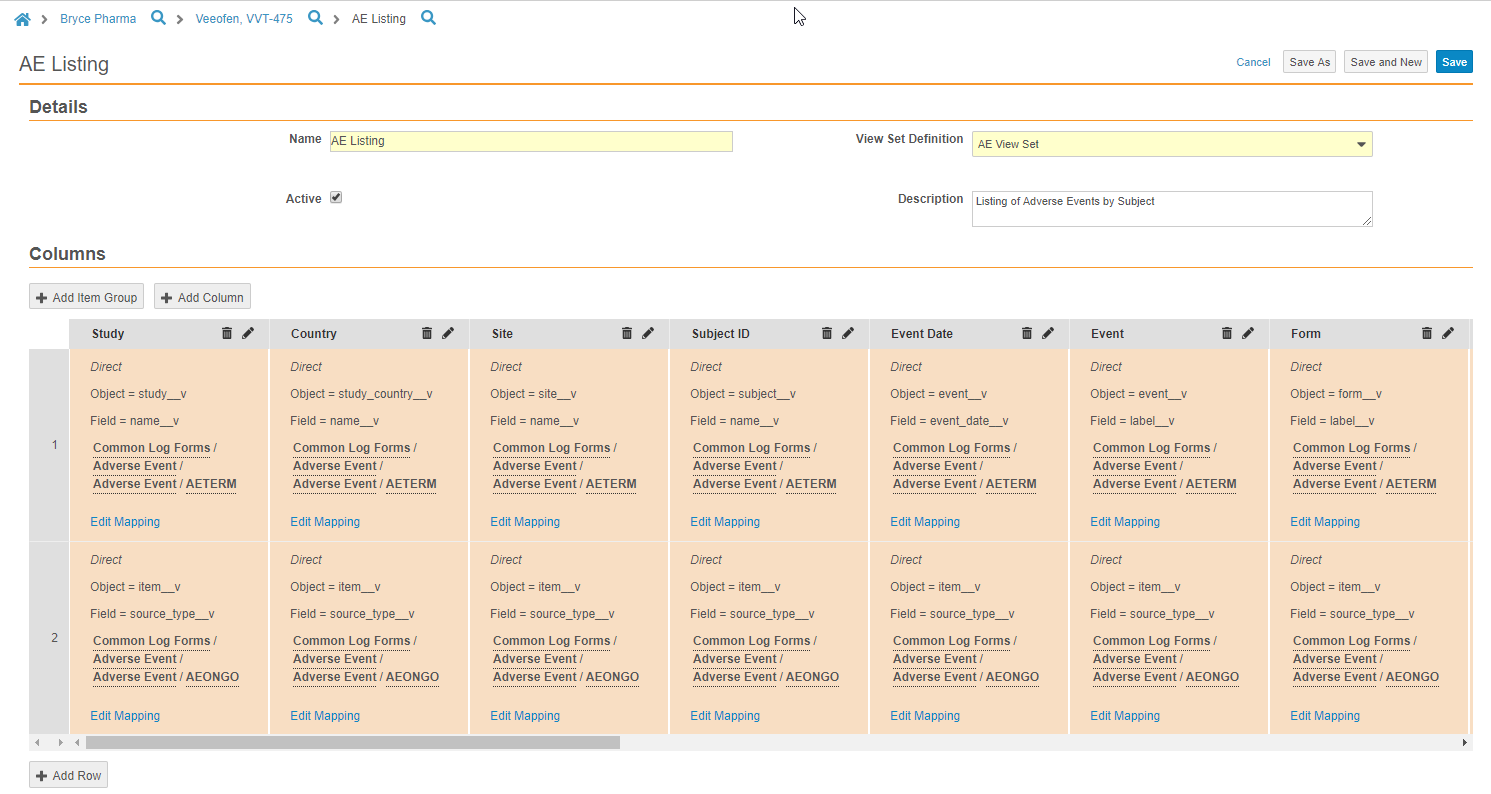
Example: Data Listing for Source Data Review
You can use views to create a data listing for quick source data review outside of the Vault EDC application. You can create a new View Set Definition for source data review, and then create View Definitions in that set to represent the Forms that require review. You can use the + Add Item Group button to quickly add entire Item Groups to your View Definition to ease view creation. You can also easily include reference columns, such as Site, Subject, and Event to improve efficiency during review.