New Features in 22R1.4
Copy Study Data, Export Package Blinding, and more...
Release Date: June 24, 2022
We are pleased to bring you the following new features in this week's release. See a summary of feature enablement for this release below. Information on developer features (REST API) is in the Developer Portal.
CDMS
Features in this section are changes that apply to all application areas of Vault CDMS.
Audit Trail Enhancement: Additional Form Entries
Description
The Form Audit Trail now includes audit entries for changes relating to the following fields:
- Study Country
- Site
Enablement & Configuration
This change applies automatically.
Data Review
Features in this section are changes to the Review tab, a working area for clinical research associates and data managers, or to review functionality within the Data Entry tab.
Assessments
The following are new features for the Assessments area of Vault EDC.
Medical Assessments: Data Changes & Reassessment
Use Case
With the reassessments feature, assessors can easily identify when assessed data has changed and when a reassessment is required.
Description
Study designers can now configure an Assessment Definition to automatically trigger a reassessment when data from the selected Items is modified post-submission. This configuration occurs in the new Reassessment phase of assessment configuration in Studio > Assessments.
When a site changes the data for one or more of the chosen Items, Vault automatically generates a new Assessment and marks it as a reassessment. The Assessments tab now includes a column for “Reassessment”. In the assessment’s header, Vault also shows the “Yes” or “No” value for Reassessment.
This feature added a new column to the Assessments sheet in the Study Design Specification, “Trigger for Reassessment”. For Items that trigger reassessment, Vault enters “Yes” in this column. For Items that don’t trigger reassessment, Vault enters “No” in this column.
Enablement & Configuration
Reassessment functionality is automatically available, but a study designer must first configure an Assessment to use reassessments before this feature is exposed to assessors.
Study Design & Configuration
Features in this area apply to Studio, the study design and configuration area for Vault EDC.
Updated Handling for Dynamic Event Group Deletion
Description
With this release, Vault no longer deletes a dynamic Event Group when the only dynamic Event present in it is deleted.
Enablement & Configuration
This change applies automatically.
Dynamic Rule Enhancement: Better Support for PPC Changes
Description
Vault now ignores the results of inactive dynamic rule definitions. If a rule is marked as Inactive after it has been run, and another dynamic rule attempts to add or remove the same targeted objects, Vault is now able to execute the new rule without interference from the inactive rule’s results.
Study designers should be aware of this change, but there is no visible change or action required. This enhancement helps improve performance and prevent issues during rule execution.
Enablement & Configuration
This change applies automatically.
Study Administration
Features in this section apply to EDC Tools, a study-level administration area for Vault EDC.
Amendment Preview: Copy Study Data
Use Case
This feature allows lead data managers the opportunity to preview the result of an amendment on actual study data.
Description
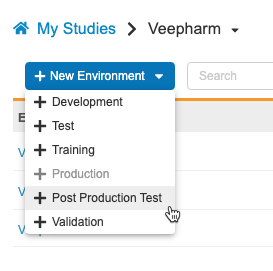
Study data, like execution data and restricted data if applicable, can be copied from Production. This does not include CDB or custom object data. Data copied to the Post Production Test (PPT) environment cannot be merged; subsequent Copy Study Data actions will first remove any existing data.
As part of this feature, we renamed the UAT environment type as TEST. Existing Environments will continue to have “_UAT” appended, but new Environments will have “_TST”. The new environment action is now labeled + Test.
Enablement & Configuration
Contact Veeva Support to enable this feature.
Learn More
Study Data Extract: Data Type & SAS Type Changes in the 22R2 Version
Description
In the 22R2 Version of the Study Data Extract, the following changes were made to fix incorrect data types:
- The column CASEBDEF has a SAS Type of numeric in the SYS_EVT, SYS_SITE, and SYS_SUB datasets.
- The column MANUAL has a Data Type of boolean in the SYS_Q dataset.
- The column NSUBMITS has a Data Type of integer and a SAS Type of numeric in the SYS_ASM dataset.
- The column IGSEQ has a Data Type of integer and a SAS Type of numeric, the column INACBYSYS has a Data Type of boolean, and the column LASTINACDT has a Data Type of datetime and a SAS Type of numeric in the SYS_PD dataset.
- The column LABMODIFIER has a Data Type of boolean in the SYS_LABRANGES dataset.
- The columns LABMODIFIER and LABSYSMANAGED have Data Types of boolean, and the column LABPREC has a Data Type of integer and a SAS Type of numeric in SYS_ANALYTES
Enablement & Configuration
This change applies automatically to the 22R2 version of the Study Data Extract job output.
Labs
Features in this section are new features for the Labs module of Vault EDC.
Versionless Codelist & Unit Definitions for Labs
Use Case
Lab units and codelists no longer need to be synced with Studio, and they can instead be managed directly from the Lab module. This avoids unnecessary deployments and retrospective amendments due to lab unit updates, which causes an artificial increase of caseboooks.
Description
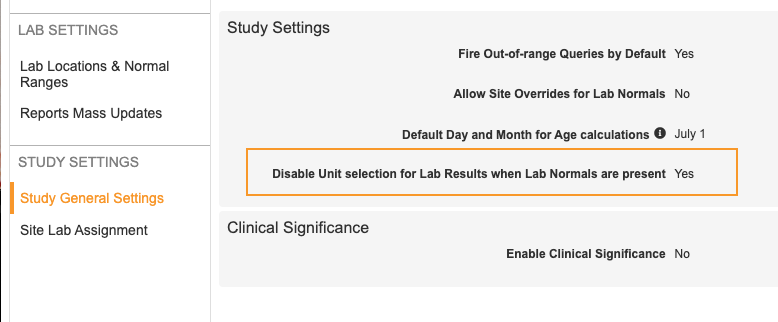
Lab Units and Lab Codelists are now managed solely in the Local Labs module (Labs tab). Changes to the Standard Unit, Conversion, and increases to Precision and Length are allowed after deployment to production (post go live).
As part of this feature, we added the ability to disable unit selection for lab results when normals aren’t present. The Disable Unit Selection for Lab Results setting is available in Labs > Study General Settings for study-level configuration, and in Admin > Business Admin > Vault Configurations for vault-level configuration. By default, this is set to No. When set to Yes, sites can’t change the Lab Result Unit in Data Entry. When set to No, sites can change the Lab Result Unit.
Enablement & Configuration
This feature is automatically available for new Studies. Existing Studies must undergo a migration process to use this feature. Studies must be on Data Model V2 before the Lab Migration can occur.
Clinical DataBase (CDB)
The following are new features for the CDB application, the Vault CDMS solution for data cleaning and reporting.
Availability: Clinical DataBase (CDB) is only available to CDB license holders. Contact your Veeva Services representative for details.
Export Package Blinding
Use Case
This feature increases flexibility in which type of user can schedule export package generation. For example, an unblinded user can now schedule the delivery of an unblinding package to an FTP server.
Description
Prior to this release, the blinding of an export package upon delivery or download was based on whether the user had the Restricted Data Access permission. With this release, package blinding is user-defined in the properties of the Export Definition. The default setting is Blinded. A user who has edit access on the Export Definition (the owner of the Export Definition or a vault owner) can change this setting to Unblinded. At package generation, CDB uses the blinding setting to determine blinding. If a package is blinded, users without restricted data access will be unable to download it.
Enablement & Configuration
This feature is enabled automatically. CDB sets any Export Definitions created prior to this release as blinded. To generate unblinded packages for these definitions going forward, a user with edit access should update it to Unblinded in the properties. Export packages generated prior to this release will maintain their existing behavior (blinding is determined by the user’s access to restricted data).
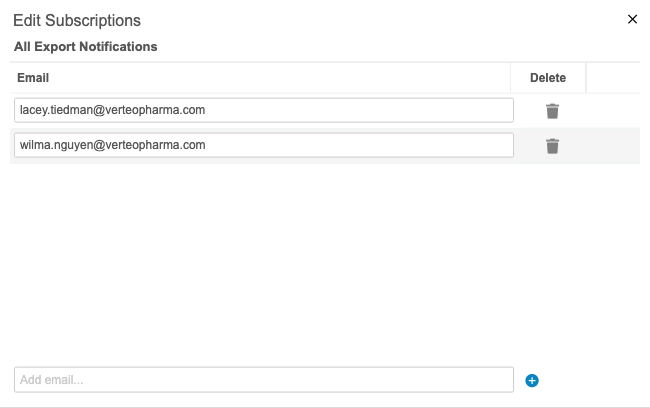
Export Package Notifications
Use Case
This feature improves monitoring for exports in CDB. Export users have insight into the export package metrics. Users can keep track of packages without navigating to the CDB Exports page.
Description
Users can receive notifications about export packages. For example, they can choose to receive an email when a package fails to generate or delivery to an FTP destination fails. An admin user (a user with the View Admin permission) can add users as subscribers to different statuses from Admin > Export Notifications. The following status options are available for notification subscriptions: All Statuses, Package Complete, Package Error, Package Delivered, and Package Delivery Failed.
The notifications show different messages based on the status:
| Status | Message |
|---|---|
| Complete | Export package has successfully generated. |
| Error | Package failed to generate due to an error with the package. Please see log for issues. |
| Delivery Failed | Package successfully generated but failed to deliver. |
| Delivered | Package successfully generated and delivered |
Enablement & Configuration
This feature is enabled automatically, but an admin user must add users as subscribers for them to receive notifications.
Automated Checks
Use Case
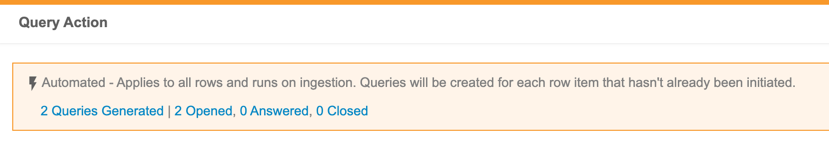
The Automated Checks feature provides a way for data managers to automate parts of their cleaning processes, monitor it, and adjust as necessary, so that they can focus their efforts on discrepancies that truly need detailed review.
Description
Users with the Create Listings permission can now create Checks. Within a Check, a user can define a query action on each Event Date or Item that exists on the check. CDB then opens a query against new records as they are captured by the Check. When a row is no longer captured by the Check (indicating that the issue has been resolved), CDB automatically closes the query.
This feature adds a new tab to CDB, Checks, which is accessible from the navigation drawer () and the Study menu. The review dashboard also includes a Checks widget.
Enablement & Configuration
This feature is enabled automatically, but CDB users must create checks for them to run.
Export Audit Logs
Use Case
This feature improves monitoring for exports in CDB. Users can access audit logs for events such as when a user downloaded a package, what listings was an export definition created with, and when listings were generated as part of what package.
Description
With this release, we added an Audit Logs page to Exports. This contains audit logs for Exports (Export Definitions), Export Packages, and Export Listings. The audit log records metrics around generation and delivery time. Users can choose the Log Type and Date Range of the audit event. Users can select a date range of 90 days at a time.
For Export Definitions, the Audit Log tracks the following events:
- Export definition created and what listings it was created with (the Audit Log shows the number, download the CSV for a list)
- Selected Listings modified and what listings were removed/added (the Audit Log shows the number, download the CSV for a list)
- Export definition deleted
- Export schedule created
- Export schedule modified
- Export schedule deleted
- Export delivery enabled
- Export delivery FTP connection modified
- Export delivery disabled
The Export Definitions log type includes the following columns:
- Log Type
- Timestamp (GMT)
- Export ID
- Export
- Type
- User
- Event
For Export Packages, the Audit Log tracks the following events:
- Package generation initiated
- Package downloaded
The Export Package log type includes the following columns:
- Log Type
- Timestamp (GMT)
- Export ID
- Export
- Type
- Package ID
- Package
- User
- Event
For Export Listings, the Audit Log tracks the following events:
- Export Listing generated in a package
The Export Listings log type includes the following columns:
- Log Type
- Timestamp (GMT)
- Export ID
- Export
- Type
- Package ID
- Package
- Export Listing ID
- Export Listing
- User
- Event
For export packages, users can also download a package log as a TXT file for an export package from Exports > Packages, which includes metrics around generation and delivery time. The file name format is “{StudyName}-{ExportDefinitionName}-Export_Log-{yyyy}-{mm}-{dd}T{HH}_{mm}_{ss}.csv”, with the datetime as the date and time the file was downloaded in GMT. This log includes the following information:
- Export Definition Type
- Listings
- Export Definition Created Date
- Export Definition Created By
- Schedule
- Scheduled By
- Delivery
- Package Format
- Package Processed By
- Package Process Start Time
- Package Process Completion Time
- Package Process Duration
- Delivery Start Time
- Delivery Completion Time
- Delivery Duration
- Size
- Oldest Date Applied
- Latest Date Applied
Enablement & Configuration
This feature is enabled automatically. Note that the audit log begins recording events at the time of release.
3rd Party Data Import Enhancements
Description
This feature includes the following enhancements:
- When a file contains a column without a header name, CDB generates the F-016 warning.
- If a third party data package doesn’t contain any data (CSV files only have a header row), CDB creates a blank Core Listing for the source.
Enablement & Configuration
This feature is automatically enabled.
CDB Usability Enhancements
Description
Users are now able to filter listings and checks with case insensitivity via contains, doesn’t not contain, starts with and ends with operators for string fields.
Enablement & Configuration
These changes apply automatically.
Role Management & Security
Features in this section are enhancements to the System Tools > Role Management and System Tools > Users areas, as well as changes to standard Study Roles, security, and access control in Vault CDMS.
Study Role Enhancements
Description
With this release, we made the following changes to standard Study Roles:
- Assigned the new Copy Study Data to PPT permission, which controls the ability to copy study data into PPT environments, to the CDMS Super User role
- Assigned the new Manage Study Settings permission, which controls the ability to edit study settings from EDC Tools > Study Settings, to the CDMS Super User, CDMS Lead Data Manager, CDMS Study Designer, CDMS Librarian, and CDMS User Administrator roles
Enablement & Configuration
These changes apply automatically to standard Study Roles.
Additional Fields Available in System Tools > Users
Use Case
User administrators can now edit these fields from System Tools > Users, instead of needing to modify these fields from Admin > Users & Groups.
Description
The following fields are now available for editing in System Tools > Users: Domain Admin, Service Availability Notifications, Product Announcement Emails, and Status. These fields can also be modified via user import.
Enablement & Configuration
This feature is automatically enabled.
Course Status in Training Report
Use Case
The existing User Training Report did not adequately delineate between curricula that were 100% complete, curricula that had a new course added, or curricula where a course was split. This new column provides more precision in evaluating a user’s training status and furnishes a clear verification of study access eligibility for audit purposes.
Description
With this release, we provide more clarity in the User Training Report by adding a new column for the user’s course status. The Course Status column will have the following values depending on the relevant scenario:
- Completed
- Optional, Not Completed
- Not Completed
- Previous Course Completed
- New Course Detected
Enablement & Configuration
This feature is only available to certain early adopters. Contact your Veeva Services representative for details.
My Training Tab
Use Case
Previously, a user’s training requirements, training status, and study access could be unclear without simultaneously inspecting the external training system transcript. In addition, some site users may have seen a vague error message when accessing the platform before all training requirements were met. The addition of the My Training Tab eliminates these ambiguities by providing a central location to view and manage training and study access.
Description
With this release, we introduced a new main navigation tab, My Training, that provides applicable users a convenient and straightforward way to manage their training requirements and study access. From the My Training tab, users can quickly determine whether they have outstanding training for a given role, access the learning portal in a new tab, and fetch their individual status from the learning portal in real time.
Enablement & Configuration
This feature is enabled automatically in Vaults using the integration.
Feature Enablement Summary
| Feature Name | Configuration | Dependencies | Day 1 Impact to Primary Users | Users with Day 1 Visibility |
|---|---|---|---|---|
| CDMS | ||||
| Audit Trail Enhancement: Additional Form Entries |
|
|
||
| Data Review | ||||
| Assessments | ||||
| Medical Assessments: Data Changes & Reassessment |
|
|||
| Study Administration | ||||
| Amendment Preview: Copy Study Data | Support | Data Model V2 | ||
| Study Data Extract: Data Type & SAS Type Changes in the 22R2 Version |
|
|||
| Study Design & Configuration | ||||
| Updated Handling for Dynamic Event Group Deletion |
|
|||
| Dynamic Rule Enhancement: Better Support for PPC Changes |
|
|||
| Labs | ||||
| Versionless Codelist & Unit Definitions for Labs | Vault Admin | Data Model 2 |
|
|
| Vault CDB | ||||
| Export Package Blinding | CDB |
|
||
| Export Package Notifications | CDB |
|
||
| Automated Checks | CDB |
|
||
| Export Audit Logs |
|
|||
| 3rd Party Data Import Enhancements |
|
|||
| CDB Usability Enhancements |
|
|||
| Role Management & Security | ||||
| Additional Fields Available in System Tools > Users |
|
|||
| Course Status in Training Report | Support | LMS Integration | ||
| My Training Tab | LMS Integration | |||
Enablement Legend
- Configuration: This field lists the location(s) where configuration for this feature occurs, for example, "Studio" or "EDC Tools". "Support" indicates that this feature must be enabled by Veeva Support, and "Vault Admin" indicates that configuration must be performed by a Vault Owner in the vault's Admin area.
- Dependencies: This field lists any dependencies required to use this feature, for example, Labs or Expression Engine V2. The other columns assume that the dependencies are enabled/in use.
- Day 1 Impact to Primary Users: This feature is visible and available to one or more primary user teams (Site Users, Clinical Team, and Coders) on day 1. Otherwise, this feature is either only visible to study designers or administrator users, it requires configuration before it is visible to primary users.
- Users with Day 1 Visibility: This feature is visible to these users on day 1 if no configuration occurs.