New Features in 21R1.3
Delayed Execution Rules, SDS Updates, and more...
Release Date: June 4, 2021
We are pleased to bring you the following new features in this week's release. See a summary of feature enablement for this release below. Information on developer features (REST API) is in the Developer Portal.
Data Entry
Features in this section are changes to the Data Entry tab, a working area for investigators and clinical research coordinators to enter study execution data.
EDC General Enhancements
Use Case
The View Summary dialog provides an easy way to view multiple repeating Form instances within a single view and easily jump to any given instance. Defaulting the unit value when only one exists allows for Data Entry units to be more efficient.
Description
With this release, Studies with the “Repeating Form Expansion” setting enabled have the ability to click “View Summary” next to a Repeating Form name in the Review tab to view a table that contains all the instances of that Repeating Form. When viewing the summary, users can click into any row to auto-scroll the Form panel of Review to the selected Form instance. This feature allows users to quickly navigate to a specific instance rather than scrolling up or down on the page.
In Data Entry, unit items that only contain one unit will now default to that unit. Users will no longer need to manually select the single unit value each time they encounter a unit item with only one option.
Enablement & Configuration
The Repeating Form Expansion setting must be enabled for the Study. Unit defaulting will automatically apply to any study with a unit that only contains one option.
Editable Grids
Use Case
Editable Grids/Tabular Data Entry allows for site users to more efficiently enter data into repeating item groups.
Description
Study Designers now have the ability to configure a repeating item group to appear in Data Entry in an Editable Grid format. Editable Grids allow for site users to efficiently enter data in a tabular, spreadsheet-like, format. Tabular data entry enables the user to quickly enter and view data across multiple items and item group instances without the need to continuously scroll up and down the page. This updated format is also an easier way to enter data for users who are accustomed to interacting with spreadsheets.
Users can access the display format for repeating item groups in the properties panel for the selected Item Group in Studio. The Editable Grid display format, when enabled in Studio, will show the item group to the site user in Data Entry in a table format, with each item displayed in a separate column. The Editable Grid item group displays the tabular format for both submitted and unsubmitted forms.
Enablement & Configuration
Study Designers can configure any new or existing repeating item group in Studio via the Item Group Properties Panel. Editable Grids are only supported on Data Entry V2 studies.
Data Review
Features in this section are changes to the Review tab, a working area for clinical research associates and data managers, or to review functionality within the Data Entry tab.
Enhanced System Query Closure Restrictions
Use Case
Sponsors can now restrict which users can close queries that were created by the System.
Description
The Query Team Restrictions feature has been expanded with this release to include system queries. Previously, Query Teams could only be applied to manual queries. Now, study designers can choose to set the Query Team that is applied to each system query via Studio’s Settings page for the given Study. System queries that are tagged with a Query Team can only be closed by users in a role on that same team. For example, a study designer can choose to tag all system queries with the Data Management team. As the system creates queries on Forms, those queries will be tagged with the Data Management team. Only roles on the Data Management team (the standard CDMS Data Manager and CDMS Lead Data Manager study roles, and any custom Study Roles assigned to that team) will have the ability to close these queries. Vault disables the Close Query button for users outside the specified Query Team, with a message to inform them that they don’t have the ability to close this query.
Enablement & Configuration
Study designers can configure this feature from Studio > Settings when Enable Team Query Restrictions is set to Yes for the Study.
Study Design & Configuration
Features in this area apply to Studio, the study design and configuration area for Vault EDC.
Aggregate Identifiers & Functions
Use Case
This feature increases the variety of rules that can be written by allowing Study Designers to consider values of an identifier as an array. An example would be being able to return the maximum of all dates entered into a repeating item group, or across multiple repeating forms.
Description
Study designers can now specify if an identifier should return an array of values, instead of distinct values. For the repeating parts of an identifier, they can use an asterisk (*) instead of a number inside the brackets to indicate that all instances of repetition should be considered in the value of the identifier.
Aggregate identifiers can’t be used standalone in an expression. They must be used within an aggregate function. Aggregate functions include:
- Sum
- Min
- Max
- First
- Last
- FindValue
These aggregate identifiers cannot be used standalone in an expression and need to be used within an aggregate function (like Sum(), Max(), …)
Enablement & Configuration
This feature is available automatically in the Rule Editor for Studies using version 2 of the expression grammar.
Cross Vault Copy
Use Case
This feature allows users to better leverage template vaults, which provide increased security for library collections.
Description
The Copy From Study dialog now allows study designers to select the vault they want to copy from, as well as the study and environment. Users can copy definitions (Event Groups, Events, Forms, Codelists, Units, and Rules across selected vaults. Previously, users could only copy from studies within the current vault. This allows users to copy definitions from template vaults or even UAT or production vaults when necessary.
Enablement & Configuration
This feature is available automatically for all Studies using automatic deployments.
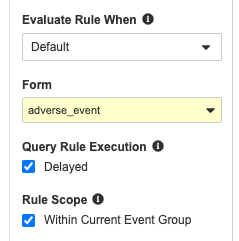
Delayed Execution for Query Rules
Use Case
With recent enhancements to rules functionality, rules may take longer to process after form submission, as they need to gather more data points across the schedule. We recommend using asynchronous rules when the expression contains a Form Link or aggregate identifier, or if the rule would result in a reciprocal evaluation, to help maintain form submission performance.
Description
Study designers can mark query-type Rules as having “delayed execution”, meaning that after a Form is submitted, the rule enters a queue for processing, instead of processing immediately after form submission. As a result, queries created by these asynchronous rules don’t display directly after the post-submission form refresh. Instead, these queries will display anytime after the application is refreshed, for example, when navigating to a different Form.
Some rule types already have this delayed behavior, such as Add Assessment, Create Protocol Deviation, Send Email, and Override Review Plan rules. These rules remain fully asynchronous, without study designers having to mark them as delayed.
In support of this feature, we added an error console to EDC Tools > Rules that displays any errors that occur during the execution of asynchronous rules. Vault keeps errors in the console for sixty (60) days.
Enablement & Configuration
This feature is available for Studies using version 2 of the expression grammar. Study designers must configure this feature for individual rules.
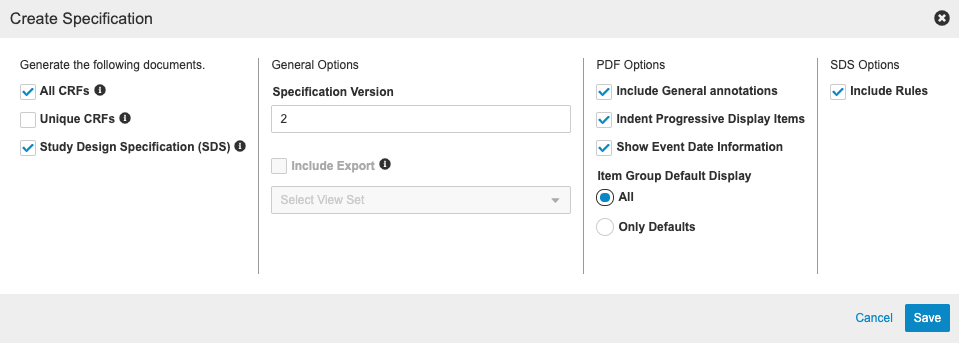
Annotated PDF Enhancements
Use Case
Enhanced usability provides more support for design validation and documentation.
Description
This release includes the following enhancements to annotated PDFs::
- The summary now includes the build number along with the casebook version
- Users can enter a specification version when creating the documents .
- There is better support for the display of default Item Groups.
- Annotations for the Codelist and Unit Names.
- Annotations for disabled rules (if this is not a progressive display study) for studies using rule v1 and studies created prior to 20R1
- Annotations for Item Groups
- Annotations for Event to include Event Date
- Annotations for SDTM at Form and Item
- Formatting changes including text color and the Form Name is now inline with the rest of the Form.
There is now also an updated dialog for annotated PDF creation.
Enablement & Configuration
These changes apply automatically.
SDS Updates
Use Case
These updates provide additional information for study designers and data management and improve usability of the specification.
Description
The SDS has been updated for general quality improvements including the following:
- Schedule tree updates - like adding names for event group and form
- Schedule Grid updates - like adding the event group
- Carrying labels down through columns like the names
- Removing the system rules from the rules tab and adding rule bindings
- Adding a local lab configuration tab
- Adding additional information to the summary page
Enablement & Configuration
This feature is available automatically.
Study Administration
Features in this section apply to EDC Tools, a study-level administration area for Vault EDC.
Allow Deletion of Sites in Production
Use Case
If a Site is created in the wrong country by mistake, users can delete that Site in Production.
Description
Users can delete a Site in EDC Tools in Production environments if there are no subjects in that Site.
Enablement & Configuration
Auto-on.
Support Multiple Site Selections in Review Plan Assignment Criteria
Use Case
Users can assign plans to selected Sites individually or as a group.
Description
With this release, we allow lead data managers to apply review plan assignment criteria to Sites or Countries as a group or individually. Lead data managers can choose to assign Review Plans for each Site, by Countries, or by Sites using Dynamic Review Plans. If you apply assignment criteria by Sites or Countries individually, then selected override plans apply to each selected Site or Country. If you apply assignment criteria by Sites or Countries as a group, then selected override plans apply on a first come, first served basis for all selected Sites or Countries.
Enablement & Configuration
A Vault Administrator must enable this feature in a study by setting Review Plan Assignment Version to 3 on the Study Configuration record.
Randomization
Features in this section are new features for the Randomization module of Vault EDC.
Send Email When Subject is Randomized Rule
Use Case
Defined user groups will be notified when a subject is randomized.
Description
With this release, users can configure an email to be sent when a subject is randomized.
Enablement & Configuration
This feature is available automatically in Studies where Randomization is enabled.
Clinical DataBase (CDB)
The following are new features for the CDB application, the Vault CDMS solution for data cleaning and reporting.
Availability: Clinical DataBase (CDB) is only available to CDB license holders. Contact your Veeva Services representative for details.
Import Monitoring & Updated Notifications
Use Case
These changes provide more detailed information about data loads that wasn’t previously available and eliminate redundant information.
Description
With this release, Workbench now only sends an email notification when data reprocessing (after a new package is imported into another source) results in changes to the issue log. Prior to this release, Workbench sent an email notification upon the completion of any reprocessing. This created a lot of noise and made it difficult for recipients to determine if there were any changes that they needed to make.
We also made the following enhancements to the data import process and UI to provide additional information about data loads:
- For each package processed, Workbench creates a log file with details about the import job. The log file contains the start and completion times for the transformation and import states of the job, as well as the duration of transformation and import. Users can download the log file, named “{StudyName}-{SourceName}-{yyyy}-{MM}-{dd}T{HH}-{mm}Z” using the import date and time in UTC, from Import > Packages.
- We updated the name of the Issue Log download file to “{StudyName}-{SourceName}-{yyyy}-{MM}-{dd}T{HH}{mm}{ss}Z.csv” to match up with the log file.
- We added the Deployed column to the package list, which displays the datetime that the source was put into use. This is useful for determining if the data was ever displayed in Workbench. If a new package for the source is imported before the original finishes processing, Workbench switches to the newest package, and so the original package’s data never displays in a listing.
Enablement & Configuration
These changes apply automatically for all imports following the release.
3rd Party Data Import Enhancements
Use Case
These changes enhance data ingestion to support additional data scenarios.
Description
With this release, the “site” key in the manifest file is optional. When the manifest file doesn’t include a “site” key, CDB expects that all Subject IDs are unique within the Study. If CDB finds a row with a duplicate Subject ID, it skips the row and records the skip in the Issue Log. Users can resolve this by updating their configuration in EDC to prevent duplicate Subject IDs.
Users can now also set a default Event for a given data load. If the data doesn’t include Event information, the manifest file can define a default Event to import the data into. This can occur at the package (source) or file (form) level. Using this event default in the manifest eliminates the need for data vendors to modify the data files to add an Event column and enter into the defaulted value for each row.
Enablement & Configuration
This feature is available automatically. Following the release, users can begin importing packages with manifest files reflecting these changes.
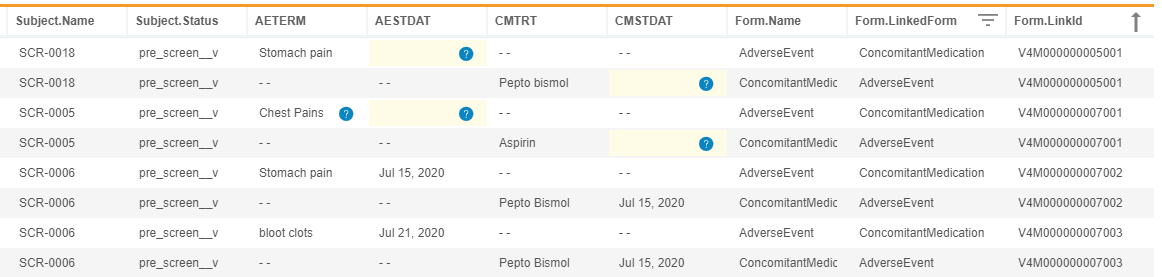
Access Form Links from Workbench
Use Case
This feature allows for the extraction of form linking data out of CDB using the raw export type. Furthermore, the new form attributes help data managers review and clean form links.
Description
With this release, CDB now imports Form Link data from EDC. Users can return the list of linked forms in a given study by entering CALL Sys_Links into the CQL Editor. The resulting dataset contains the following headers:
Study.NameSite.CountrySite.NumberSubject.NameEventGroup.NameEventGroup.SeqNbrEvent.NameForm.NameForm.SeqNbrForm.LinkCreatedDateForm.LinkID
Each row contains one side of a link. For example, if an AE form and a CM form are linked, two records display, one for each form with the same Form.LinkID. By default, the results are sorted by LinkID. The resulting CQL aren’t further filterable or sortable.
As part of this feature, we introduced new form attributes: @Form.LinkedForm and @Form.LinkId. These attributes help data managers to review and verify form links by allowing them to return columns from linked forms without using a subquery.
For example, the following CQL will return Header information, AETERM and AESTDAT from AdverseEvent form, CMTRT and CMSTDAT from ConcomitantMedication form, Form name, Linked Form Name, and LinkId from Adverse Events and Concomitant Medication forms that are linked together.
SELECT @HDR, AdverseEvent.AETERM, AdverseEvent.AESTDAT, ConcomitantMedication.CMTRT, ConcomitantMedication.CMSTDAT, @Form.Name, @Form.LinkedForm, @Form.LinkId
FROM AdverseEvent, ConcomitantMedication
WHERE @Form.LinkedForm IN ('AdverseEvent', 'ConcomitantMedication')
ORDER BY `@Form`.`LinkId` ASC
The CALL Sys_Links dataset is automatically generated and included in the raw export. The Sys_Links listing behaves the same as the other system datasets in a raw export.
Enablement & Configuration
The changes to import and CQL apply automatically, but Workbench does not add the Sys_Links listing to existing raw Export Definitions. To export Sys_Links, create a new raw Export Definition after the release.
Improvements to System Listings
Use Case
This feature eliminates the need for system datasets, such as Sys_ILB, in the raw export definition to be split into multiple files.
Description
Raw-type Export Definitions automatically contain the following auto-generated System Listings: Sys_Sites, Sys_Subjects, Sys_Events, Sys_Forms, and Sys_ILB. With this release, we’ve updated the CQL of these system datasets as follows:
| System Listing | CQL Syntax |
|---|---|
| Sys_Sites | CALL Sys_Sites |
| Sys_Subjects | CALL Sys_Subjects |
| Sys_Events | CALL Sys_Events |
| Sys_Forms | CALL Sys_Forms |
| Sys_ILB | CALL Sys_ILB |
The headers in these listings are unchanged. By default, all listings, except for Sys_Sites, are sorted by Subject.Name. By default, Sys_Sites is sorted by Site.Name. The resulting CQL isn’t further filterable or sortable.
Enablement & Configuration
These changes only apply to raw Export Definitions created after this release. To apply these changes to existing definitions, a user must edit that definition and save their changes.
Role Management & Security
Features in this section are enhancements to the System Tools > Role Management and System Tools > Users areas, as well as changes to standard Study Roles, security, and access control in Vault CDMS.
Multi-role Security (Limited Release)
Use Case
Users can have multiple roles in a study. A user may be a lead data manager, but that user can also be assigned a CRA role to preview the study as a CRA would view it in the Review tab.
Description
Vault CDMS now offers a security model that allows users to have multiple Study Roles in the same Study. A user can have up to 15 distinct roles in a vault, either across the same study or across multiple studies. This model leverages the Role Based Security feature. If you have more than one Application Role in a study, resulting access to a study is the combination of all your permission across the roles. All users will now share a common security profile, CDMS All Access.
Enablement & Configuration
Contact Veeva Services to discuss enabling this feature in your vault.
LMS Enhancements
Use Case
Users can now create a Site without needing a study design.
Description
With this release, we made the following enhancements in support of the LMS integration:
- If a Casebook Definition does not yet exist for a Study, the Active Version field on Sites is no longer required. This allows users to configure and begin training for a Study before a study design is complete.
- The Get Enrollment check now occurs every 12 hours, instead of every 24 hours. Vault checks for enrollment at 12:00 AM and 12:00 PM, based on the vault’s timezone.
- The LMS Integration feature is now enabled using the Study Settings object, instead of the study’s Study Configuration record. To enable the feature with this release, a Vault Owner must edit the “lms.enabled” record for the Study to set the Value field to “true”. For any Studies where the integration was already enabled, the “lms.enabled” record automatically has the value set to “true”.
Enablement & Configuration
These changes apply automatically.
Study Role Enhancements
Description
With this release, we made several improvements to the available permissions and standard Study Roles.
We added the following new permissions:
- View Users: Controls the ability to view Users from Tools > System Tools > Users
- Assigned to the standard CDMS User Administrator role and any custom Study Roles that have the Manage Users permission assigned
- View Query: Controls the ability to view Queries in EDC
- Assigned to the following standard Study Roles and any custom Study Roles that have the Open Query, Answer Query, Close Query, or Close All Query permissions assigned:
- CDMS Clinical Coder
- CDMS Clinical Coder Administrator
- CDMS Clinical Coder Manager
- CDMS Clinical Research Associate
- CDMS Data Manager
- CDMS Lead Data Manager
- CDMS Principal Investigator
- CDMS Sub Investigator
- CDMS Study Designer
- CDMS Librarian
- Assigned to the following standard Study Roles and any custom Study Roles that have the Open Query, Answer Query, Close Query, or Close All Query permissions assigned:
- View Study Sites: Controls the ability to view Sites in Tools > EDC Tools > Sites
- Assigned to the standard CDMS Lead Data Manager, CDMS Study Designer, CDMS Librarian, and CDMS User Administrator roles and any custom Study Roles that have the Edit Study Sites permission (formerly known as Manage Study Sites) assigned
We granted the View Casebook permission to all Study Roles, both standard and custom, that have the Data Entry permission assigned.
We granted the View Casebook, Schedule Reports, and Reports Dashboards Tab permissions to the CDMS Auditor Read Only study role.
We relabeled the following permissions:
- Manage Study Sites as Edit Study Sites
- Manage Users as Edit Users
- View Query (existing permission for viewing queries in Workbench) as View CDB Query
We created the following new standard Study Roles:
- CDMS Super User: Users with this study role can access all areas of the application and perform all actions within those areas. This role is in the Administration query team. This role is only available in non-production environments.
- CDMS API Read Only: Users with this study role have read-only access to the EDC API. This role is in the Other query team. This role has the following permissions:
- API Access
- View Query
- View Study Sites
- View Casebook
- View Code
- CDMS API Read Write: Users with this study role have read and write access to the EDC API. This role is in the Other query team. This role has the following permissions:
- API Access
- Add Casebook
- View Casebook
- Data Entry
- View Code
- View Study Sites
- Edit Study Sites
- View Query
- Open Query
- Answer Query
- Close Query
Because these Permission Sets are no longer used by standard Security Profiles, we appended “DEPRECATED” the Labels of the following Permission Sets:
- CDMS Definition Objects Read Only
- All Access Actions
- Base CRA Permissions
- Base Data Manager Permissions
- Base EDC User Permissions
- Base Site User Permissions
- Base Standard Template Report Permissions
- Business Administrator Actions
- EDC Clinical Coder
- EDC Clinical Coder Administration
- EDC Investigator Permission
- EDC Reviewer Permissions
- EDC Study Tools Permissions
- External User Actions
- Full User Actions
- Monitor User Actions
- Read-only User Actions
- Site User Actions
- System Administrator Actions
Enablement & Configuration
These changes apply automatically, with no additional configuration required.
Relabeled CDB's "View Query" Permission as "View CDB Query"
Description
With this release, we relabeled the CDB permission, View Query, as View CDB Query, to prevent confusion with the new permission for EDC queries, View Query.
Enablement & Configuration
The label change applies automatically.
Integrations
Features in this section are new integrations with Vault CDMS or enhancements to existing integrations.
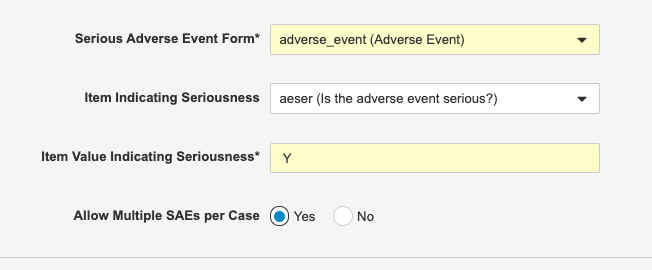
Multiple SAEs per Safety Case
Use Case
Combining SAEs into a single Safety Case reduces the need for manual data entry in the safety system..
Description
Safety Link can now be configured to include multiple Serious Adverse Events (SAEs) in a single Safety Case. This is helpful when capturing a series of related SAEs. For example, if a subject was hospitalized for a heart attack and then, a week later, had a stroke during the same hospital visit, both of those SAEs would be included in the same Safety Case.
Enablement & Configuration
Safety administrators must enable this feature by setting Allow Multiple SAEs per Case to Yes from Tools > EDC Tools > Safety Configuration.
Safety Integration Enhancements
Use Case
These updates offer enhanced integrations with third party safety systems.
Description
Safety Link now offers the ability to indicate that the Investigational Product is blinded and configurable to use a NullFlavor of 99999999 for coding.
Enablement & Configuration
Safety administrators can configure these options in Tools > EDC Tools > Safety Configuration.
Migrations Product Toolkit: Loader (Limited Release)
Use Case
Organizations can now explore feasibility of, and conduct migrations from, supported source EDC systems to Vault CDMS.
Description
The Vault CDMS Migrations toolkit is a set of tools designed to help users migrate data from various platforms into Vault CDMS EDC. This set of solutions assists users in rebuilding existing study elements (Builder), loading source data (Loader), and verifying the process (Validator). With this release, Veeva Services now has the ability to use Loader to perform various semi-automated study migration operations, including EDC subject creation, event creation, form creation, and item value population, along with support for basic transformations during the ingestion process.
Enablement & Configuration
Contact Veeva Services to discuss migrations for your organization and the appropriate transition strategy based upon your needs. In the current release, this feature is only available to Veeva Services, as they will perform any migrations on your behalf.
Feature Enablement Summary
| Feature Name | Configuration | Dependencies | Day 1 Impact to Primary Users | Users with Day 1 Visibility |
|---|---|---|---|---|
| Data Entry | ||||
| Editable Grids | Studio | Data Entry V2 |
|
|
| Data Review | ||||
| Enhanced System Query Closure Restrictions | Studio | Query Team Restrictions |
|
|
| Randomization | ||||
| Send Email When Subject is Randomized Rule | Studio | Randomization |
|
|
| Study Design & Configuration | ||||
| Aggregate Identifiers & Functions | Studio | Expression Grammar V2 |
|
|
| Cross Vault Copy | Automatic Deployments |
|
||
| Delayed Execution for Query Rules | Studio | Expression Grammar V2 |
|
|
| Annotated PDF Enhancements |
|
|||
| SDS Updates |
|
|||
| Study Administration | ||||
| Allow Deletion of Sites in Production |
|
|||
| Support Multiple Site Selections in Review Plan Assignment Criteria | Vault Admin |
|
||
| Vault CDB | ||||
| Import Monitoring & Updated Notifications |
|
|||
| 3rd Party Data Import Enhancements |
|
|||
| Access Form Links from Workbench |
|
|||
| Improvements to System Listings | Workbench |
|
||
| Role Management & Security | ||||
| Multi-role Security (Limited Release) | Support | |||
| LMS Enhancements |
|
|||
| Study Role Enhancements |
|
|||
| Relabeled CDB's "View Query" Permission as "View CDB Query" |
|
|||
| Integrations | ||||
| Multiple SAEs per Safety Case | EDC Tools |
|
||
| Safety Integration Enhancements | EDC Tools |
|
||
| Migrations Product Toolkit: Loader (Limited Release) | Support | |||
Enablement Legend
- Configuration: This field lists the location(s) where configuration for this feature occurs, for example, "Studio" or "EDC Tools". "Support" indicates that this feature must be enabled by Veeva Support, and "Vault Admin" indicates that configuration must be performed by a Vault Owner in the vault's Admin area.
- Dependencies: This field lists any dependencies required to use this feature, for example, Labs or Expression Engine V2. The other columns assume that the dependencies are enabled/in use.
- Day 1 Impact to Primary Users: This feature is visible and available to one or more primary user teams (Site Users, Clinical Team, and Coders) on day 1. Otherwise, this feature is either only visible to study designers or administrator users, it requires configuration before it is visible to primary users.
- Users with Day 1 Visibility: This feature is visible to these users on day 1 if no configuration occurs.