Clinical Data Applications Overview
Veeva’s Clinical Data Platform provides a unified, end-to-end solution for the collection and management of clinical trial data. Its robust connectivity facilitates regulatory compliance, streamlines trial administration, and enables faster identification of the data that drive critical decisions. With a seamlessly integrated product suite, systems communicate freely to prevent silos and improve efficiency, helping to reduce the time to market for new medications.
Veeva EDC
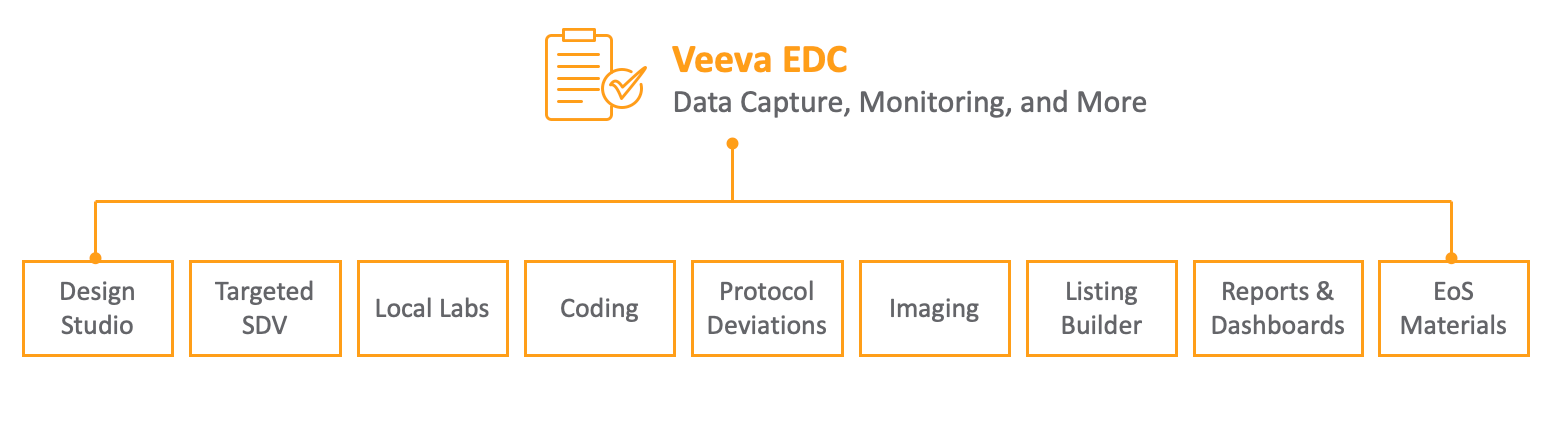
Veeva EDC is a modern, flexible electronic data capture system that is designed to reduce the manual and administrative burden for sites and sponsors. Veeva EDC’s modules centralize the collection and standardization of disparate types of clinical trial data. With its intuitive, role-based user interface, Veeva EDC enables efficient study design, streamlined data entry, and simplified review and monitoring processes. Localization support allows sponsors to tailor the languages of studies and UI as needed.
EDC Functions and Modules
Study Design
Veeva EDC supports the efficient and agile design of studies of any complexity through Studio, the study design module. This powerful and intuitive interface makes it possible to build study designs using an intuitive drag-and-drop interface to easily create study objects and case report forms (CRFs). Study designers can also easily reuse forms from Library collections or previous studies. Edit Checks, User Defined Rules, and Comparison Rules can be implemented with no custom programming needed, greatly reducing study build times and making it possible for study designers without software programming expertise to create a complete study. When a study needs to be amended after it is in production, Veeva EDC can support post go-live amendments, with no migrations needed.
Data Entry
Veeva EDC’s Data Entry UI is clean, structured, and easy for site staff to use. Its simplified navigation makes it easy to find and respond to queries. The intuitive layout and logical Casebook structure make navigating between Subjects and Forms easy, reducing data entry time. Real-time edit checks and data validation notify users of missing or inconsistent data, reducing transcription errors and improving efficiency. Comprehensive audit trails track data entry and changes ensuring regulatory compliance.
Monitoring and Review
Veeva EDC simplifies clinical trial monitoring and review to help ensure the integrity of clinical trial data. Customizable Review Plans allow configuration of the Study to only require SDV or DMR on the Items that need it, saving monitoring and data management users’ time. Veeva EDC offers a number of standard reports and dashboards that provide visibility into key study metrics from the Reports tab.
Veeva EDC Modules
Veeva EDC offers a number of modules and features that extend its capabilities and support specific clinical trial needs.
- Local Labs: provides easy access to centralized lab locations, normal ranges, and an Analyte Library, seamlessly integrated into Veeva EDC.
- Veeva EDC Imaging: allows Imaging data gathered in the course of your Study to be shared across all CDMS modules.
- Data Loader: eliminates manual data entry for large scale test results, instead importing data and automatically entering it into configured Forms and their data collection Items.
- Study Grade: reviews and grades studies based on the study’s adherence to best practices.
- Veeva Coder: a tool for the medical coding of terminology used in clinical trials that transfers casebook data and coding queries in real time, with no reconciliation necessary.
- Medical Assessments: supports the supplemental assessment and review of relevant casebook data to support decision-making about trial data.
- Clinical Reporting: Clinical Reporting provides basic reporting access to EDC clinical data captured in the Vault EDC application.
- Visit Method: allows you to specify what type of visit the event can be categorized by: On Site Visit, Virtual Visit, At Home Visit, Alternative Facility Visit, or Telephone Interview.
- Veeva EDC API : enables secure integration with external systems, built on Vault Platforms API.
Veeva CDB
In modern clinical trials, clinical data managers collect data from many sources beyond EDC. Veeva Clinical Database (CDB) aggregates, cleans, and transforms clinical data from multiple sources, including third-party EDCs.
Via CDB, data managers can access the latest trial data, assess its status, and track review progress. They can log data issues on any source by using manual queries or automated checks, to communicate with data providers without switching between EDC, trackers, and emails.
Programmers can use Veeva Clinical Query Language (CQL), designed for clinical data, to access, retrieve, and transform data for reviewers in Veeva CDB or downstream.
Veeva CDB Features
CDB Study Backbone
CDB aligns diverse data sources to a common study backbone, making it easier to compare and reconcile data from different sources. Clinical Query Language (CQL) understands the clinical backbone and can work with all data types and related metadata.
Listing Builder
Veeva Listing Builder is a drag-and-drop tool that data managers use to build Listings, Checks, or Views without writing CQL queries or code. This intuitive interface helps users build and modify listings quickly while pulling data from multiple forms, visits, and data sources. Listing Builder automatically generates CQL code based on the selections made in the interface, lowering the barrier to entry and transforming clinical trial data into meaningful information.
Clean Patient Tracker
With the Clean Patient Tracker, data managers can track the cleanliness of Subjects and take direct action on data management cleaning activities. This built-in report summarizes all data collected by subject (patient), offering drill-down to patient and query data. This feature simplifies the management of data across sources and patients, eliminating the need to jump between source systems or to rely on an external spreadsheet.
Autochecks and Query Automation, Centralized Query Management
Veeva CDB automatically checks new and incoming data when it is loaded into the system from internal or third-party sources. If an error is found, a query is created automatically, reducing manual review effort. Query resolutions can also be automatically recognized and closed. The intuitive, spreadsheet-like Data Workbench lets users access all study data from a central location. All listings and queries are viewable directly from the Data Workbench, eliminating the need to log in to EDC or refer to an external tracking sheet.
Veeva CQL
Veeva CQL is a Veeva-built clinical query language that reduces the effort and technical expertise needed for data cleaning and transformation. Clinical programmers can calculate derived values and build sophisticated listings with minimal effort. This feature offers comprehensive pre-built functions, performing tasks that normally take hundreds of lines of code in just a few.
Veeva eCOA
Veeva eCOA (electronic Clinical Outcome Assessments) captures questionnaire responses directly from clinical trial patients (ePRO), clinicians (eClinRO), or patient caregivers (eObsRO) using an app or webpage.
Sponsors can manage the eCOAs through a dedicated interface. A central library allows them to retain and reuse eCOAs across all their studies. Veeva also offers localization support for surveys targeting users who speak various languages.
Sites can manage their participants and can review eCOA data and adherence via an easy-to-use access point.
Patients and caregivers complete the questionnaires using MyVeeva for Patients (native application or web), where they can also access other activities like consent or virtual visits. Once complete, the data automatically flows back into the sponsor’s environment.
Veeva eCOA is a unified system that seamlessly connects sponsors, sites, and patients across the entire eCOA process. Data collected from Veeva eCOA can flow into Veeva CDB to provide deeper insights.
Veeva eCOA Features
Interactive Design Process, Accelerated Study Build
Stakeholders can be engaged early in the design process and throughout the study build process with eCOA previews across all device types and available languages. eCOA accelerates study design by supporting a library of reusable and validated instruments, sourced from both Veeva and sponsor libraries, as well as auto-generated screenshot documents for site review and IRB/ethics packs.
Self-Service Management Tools, Version Control
Sponsors can track study progress, make rapid mid-study changes, and export data on-demand, without extra cost or delay. Veeva eCOA offers complete tracking and audit trails for instruments, translations, and amendments to support compliance at every step of the study.
Patient and Site Notifications, Guided Navigation for Sites
Veeva eCOA notifies patients of ePROs requiring completion and alerts sites if they are not completed or missed. Within the site UI, indicators clearly guide the sites through outstanding activities that need to be completed during patient visits.
Enhanced Login Options, optimized for BYOD
Veeva eCOA makes access simple and secure. Users can login with a username and password, PIN, or choose to activate biometric authentication (fingerprints and facial recognition). Patients can access eCOA using their own device, including Android, iOS, or via a web browser.
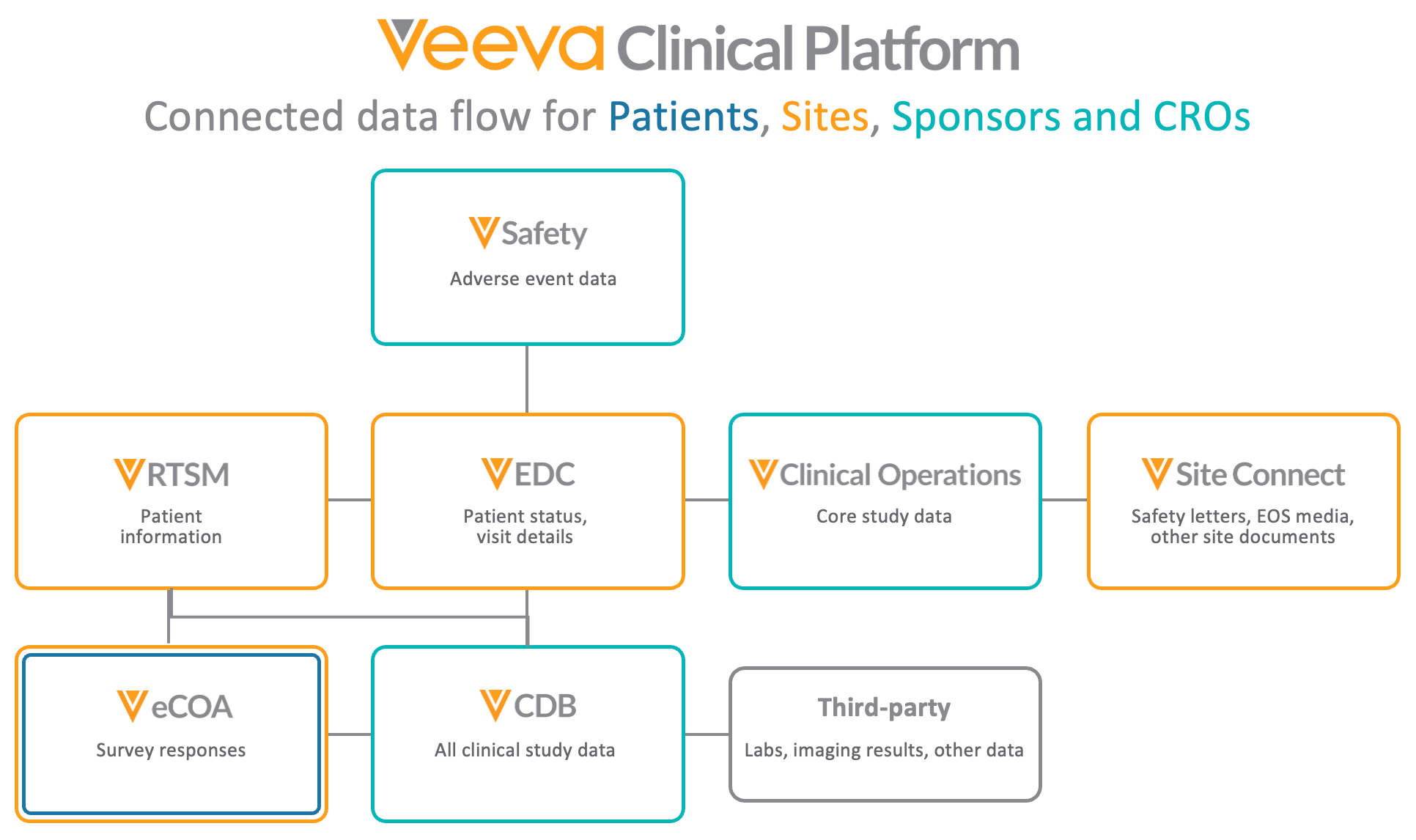
Veeva EDC and CDB Connections
Veeva EDC, CDB, and eCOA are part of the broader Veeva Clinical Platform, and offer productized connections to create powerful unified architecture that allows for data to flow across Clinical Operations vaults, Clinical Data vaults, and Safety vaults.
Veeva Clinical applications are built on the shared Vault Platform, enabling seamless connectivity across clinical data and operations. This shared infrastructure reduces manual reconciliation and provides end-to-end visibility so that teams can spend less time entering data and more time extracting the actionable insights that advance the life sciences industry.
Safety-EDC Connection
Veeva Safety is an individual case safety report (ICSR) management system that manages intake, processing, and submission of adverse events for clinical and post-marketed products, streamlining adverse event management. Veeva Safety integrates with EDC via Safety Link to automate the transmission of safety related case data. This connection ensures accurate, timely SAE reporting while reducing site burden and manual transcription errors.
Through the Safety-EDC Connection, synchronization of data between Veeva EDC and Veeva Safety is managed and maintained with mapped data fields. Organizations can easily add, modify, or disable default fields, simplifying setup and configuration.
Clinical Operations-EDC Connection
Veeva CTMS , part of the Clinical Operations platform, provides end-to-end study management and monitoring capabilities for insourced and outsourced trials. The Veeva CTMS connection with Veeva EDC supports monitoring and payments (via Veeva Payments ), and offers real-time visibility into study statistics such as enrollment status. Site staff can navigate directly to casebooks in EDC from within Veeva CTMS, without logging in separately.
The Clinical Operations-EDC Connection enables the seamless flow of information by automatically exchanging new records and updates to existing records in a fast, streamlined way through the Veeva Vault platform. Field mappings bridge the terminology differences between EDC and CTMS such as subject events and subject visits.
Veeva RTSM
Veeva RTSM is a reliable and user-friendly randomization and trial supply management solution designed to simplify complex processes and expedite clinical trials. Through the secure, consistent, repeatable connection with Veeva EDC, data entered in the Veeva RTSM system is sent to Veeva EDC in real time via a REST API, and automatically creates Subjects and Events in EDC, reducing the need for duplicate data entry by site staff and increasing efficiency.