Performing SDV
Clinical research associates (CRAs) can perform source data verification (SDV) after a form is submitted to ensure that any data collected by a site is accurate. SDV involves manually comparing data from the source to data entered in Veeva EDC. You can perform SDV from within the Review tab. For help with DMR, see Performing DMR.
Prerequisites
To use the Additive Review feature (perform DMR on Event Dates and Items that don’t require review), a study designer must enable it for your Study.
Before you can perform SDV on study data, the following configurations are required:
- A study designer creates at least one Review Plan using the SDV review task.
- A lead data manager assigns the Review Plan to the Site.
Users with the standard CDMS Clinical Research Associate study role can perform the actions described above by default. If your organization uses custom Study Roles, your role must have the following permissions:
| Type | Permission Label | Controls |
|---|---|---|
| Standard Tab | Review Tab | Ability to access the Review tab |
| Functional Permission | View SDV | Ability to view SDV status |
| Functional Permission | Edit SDV | Ability to perform SDV |
If your Study contains restricted data, you must have the Restricted Data Access permission to view it.
Learn more about Study Roles.
SDV Requirements
Depending on your vault’s configuration and your study design, you may have only SDV or DMR, or both SDV and DMR for a Study or Site. Sponsors can define which fields require SDV for a given study or site using Review Plans. See Creating a Review Plan for details. If no Review Plan exists, the Requirement Mode for all Items in your Study or Site is set no review required. SDV and DMR is required for all Forms and Items in your Study or Site.
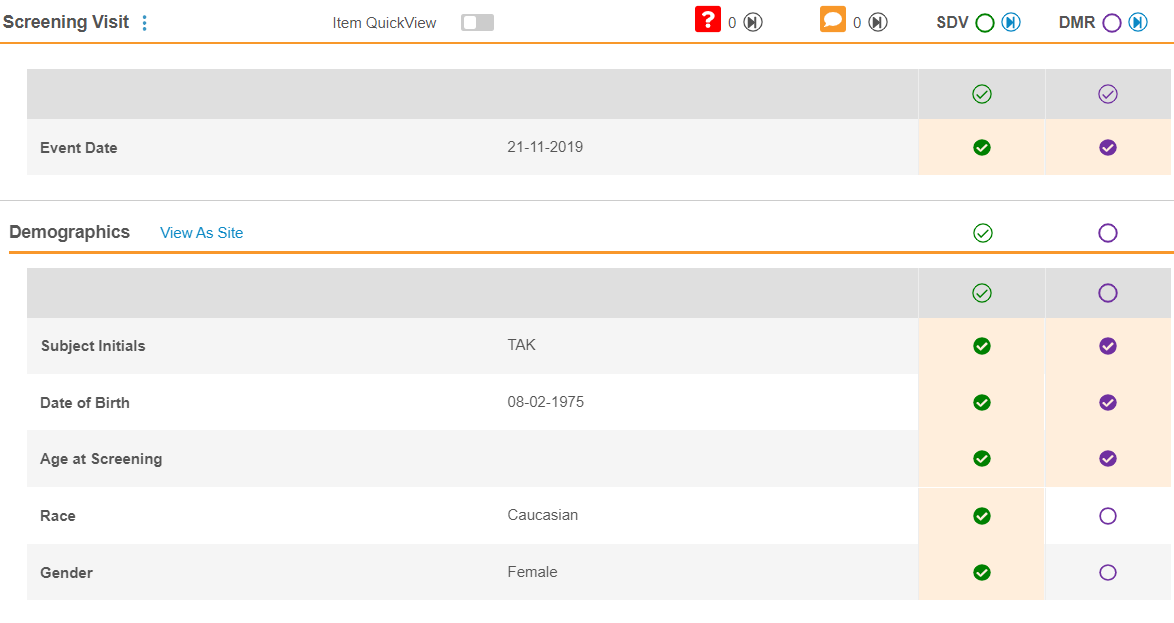
Vault displays the SDV and DMR status in the Event & Form List panel.
When you view a Form in the Content panel, you can click the SDV or DMR status icons to complete the review.
How to Perform SDV
You can perform SDV or DMR from the Content panel by clicking on the SDV status icons. As you perform SDV or DMR, Vault highlights the Items with unsaved reviews in yellow. You must click Save for Vault to save your SDV or DMR changes and update the Event and Form review statuses. You can click Discard Changes to remove your review changes since your most recent save.
SDV an Event
To perform SDV on an entire Event:
- Navigate to the Casebook containing the Event in the Review tab.
- Click to open the Event you want to review in the Schedule panel.
- Click the SDV icon in the Event Header to complete your review.
- Optional: Click Next SDV Task to quickly move to the next open SDV task.
- Click Save.
Performing SDV on an Event will apply SDV to the Event Date, Visit Method (if configured), and associated forms.
SDV an Event Date
To perform SDV on an Event Date:
- Navigate to the Casebook containing the Event in the Review tab.
- Click to open the Event you want to review in the Schedule panel.
- Click the SDV icon for the Event Date to complete your review.
- Optional: Click Next SDV Task to quickly move to the next open SDV task.
- Click Save.
SDV a Form
To perform SDV on a whole Form:
- Navigate to the Casebook containing the Form in the Review tab.
- Click to open the Form you want to review in the Schedule panel.
- Click the SDV icon in the Form Header to complete your review.
- Optional: Click Next SDV Task to quickly move to the next open SDV task.
- Click Save.
SDV an Item Group
To perform SDV on an Item Group:
- Navigate to the Casebook containing the Item in the Review tab.
- Click to open the Form containing the Item Group in the Schedule panel.
- In the Content panel, locate the Item you want to SDV.
- Click the SDV icon to complete your review.
- Optional: Click Next SDV Task to quickly move to the next open SDV task.
- Click Save.
SDV an Item
To perform SDV on an Item:
- Navigate to the Casebook containing the Item in the Review tab.
- Click to open the Form containing the Item in the Schedule panel.
- In the Content panel, locate the Item you want to SDV.
- Click the SDV icon to complete your review.
- Optional: Click Next SDV Task to quickly move to the next open SDV task.
- Click Save.
Completing SDV
Last Updated: 24R1
This video goes over how to complete source data verification (SDV) review in the Review tab of EDC.
Additive Review
From time to time, you may need to perform additional SDV on Event Dates or Items where DMR isn’t required by the review plan. You can track these reviews by using Additive Review.
When you turn on Additive Review mode, it turns on for the entire Site and not just for the Casebook being viewed. However, Additive Review is user-specific, meaning that it will only apply to the user who enables it. Once you enter Additive Review mode, Vault remains in Additive Review mode for up to 24 hours, or until you toggle Additive Review mode off.
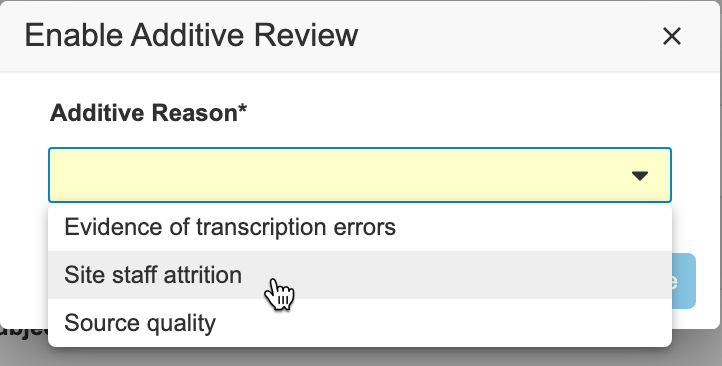
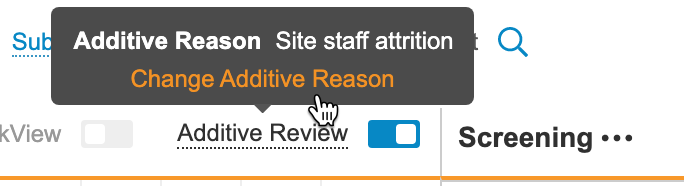
While Additive Review mode is on, you can perform SDV on any Event Date or Item within the Casebook and record a reason for the review. When you save your reviews, Vault displays a plus () icon. You can hover over that icon to show the Additive Reason.
You can perform additive reviews alongside required and optional SDV.
To perform additive review:
- Navigate to the Casebook you want to review in the Review tab.
-
Click the Additive Review toggle to turn on Additive Review mode.
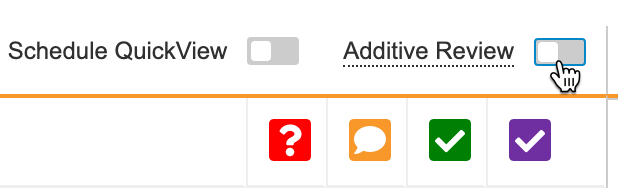
- Click Save. This starts Additive Review mode.
- Optional: To change the Additive Reason:
- Perform the necessary SDV. See instructions for each level of data above.
- When finished, click Save.
Vault will send you an email with an Additive Review summary that includes the following columns:
- Study
- Country
- Site
- Subject
- Subject Status
- Event Group
- Event Date
- Form Label
- Form Sequence
- Item Group Label
- Item Group Sequence Number
- Item Label
- Review Plan
- Last Reviewed By
- Last Reviewed Date
- Additive Reason
- Last Run of Listing
Additive Review
Last Updated: 23R1
This video shows how to perform additive review in the Review tab.
How to Clear Completed SDV
You can clear SDV to indicate that SDV should be performed again, or has been invalidated. To clear SDV, click the icon or link (for Item Groups) a second time. The icons for each item return to their unchecked state.
SDV Status Icons
Vault uses these icons to represent SDV review statuses:
| Icon | Name | Meaning |
|---|---|---|
| SDV Complete | A checked icon indicates that the required SDV is complete |
|
|
|
SDV Optional Complete | A checked icon with “optional” appended indicates that SDV is optional and completed |
|
|
SDV Optional | An unchecked icon with “optional” appended indicates that SDV is optional and incomplete |
| SDV Required | An unchecked icon indicates that SDV is required and incomplete |
|
| No Icon | SDV Not Required | No icon indicates that SDV is not required for the Event, Form, or Item. |
In the Event & Form List panel, Vault uses different icons to indicate SDV requiredness and completeness.
| Icon | Name | Meaning |
|---|---|---|
| SDV Not Started | SDV is 0% complete (not started) for the Required items or event dates. |
|
| SDV In Progress | SDV is in progress for the Event or Form, but SDV is not complete for 100% of the Items or Event Dates where SDV is Required. |
|
| SDV Complete | SDV is 100% complete for the Required items or event dates. |
|
| Planned | This Event is still in the Planned status, and so there is no associated Review State for it or its Forms. |