Creating Study Progress Listings Reports
From EDC Tools > Jobs you can schedule Study Progress Listing jobs to to be populated in the Reports tab. As part of the job, Vault sends the listing data to the appropriate report. In the Reports tab, there are standard templates that match the output of the listing from EDC Tools. Users with access to Reports can create their own version of the report based on the standard template, which they can customize by adding filters or by adding or removing columns. Users can also create dashboards based on the reports. Learn more about how to create reports, filter reports, and create dashboards. User access to report data is automatically restricted by the Study, Site, and restricted data access permissions.
The following Study Progress Listing jobs are available as Reports:
- Event Progress Listing
- Form Progress Listing
- Query Detail Listing
- Subject Progress Listing
V2 Study Progress Listing Reports: In the 24R1 release, Vault made V2 Study Progress Listing Reports available. Only V2 Study Progress Listing Report templates will be updated in future releases. We recommend that you use the V2 Study Progress Listing Report if it is available for your study.
Prerequisites
Users with the standard CDMS Lead Data Manager study role can create ad hoc and scheduled jobs by default.
If your vault uses custom Study Roles, you must have a Study Role with the following permissions:
| Type | Permission Label | Controls |
|---|---|---|
| Standard Tab | EDC Tools Tab | Ability to access the EDC Tools tab |
| Functional Permission | Manage Jobs | Ability to create, edit, and delete scheduled jobs |
If your Study contains restricted data, you must have the Restricted Data Access permission to view it.
Learn more about Study Roles.
Study Progress Listing Report Types
From EDC Tools > Job Schedule, you can initiate Study Progress Listing jobs to export Study data as Reports.
Summary
The table below summarizes the Study Progress Listing job types available as Reports:
| Job Type | Description |
|---|---|
| Event Progress Listing |
Generates a list of all Events in a Study with status details and operational information. |
| Form Progress Listing |
Generates a list of all Forms in a Study with status details and operational information. |
| Query Detail Listing |
Generates a list of all Queries in a Study with status details and operational information. |
| Subject Progress Listing |
Generates a list of all Subjects in a Study with status details and operational information. |
| Form Progress Versioned Extract |
Generates a list of all Forms in a Study with status details and operational information according to the selected version. |
| Event Progress Versioned Extract |
Generates a list of all Events in a Study with status details and operational information according to the selected version. |
| Subject Progress Versioned Extract |
Generates a list of all Subjects in a Study with status details and operational information according to the selected version. |
| Query Detail Versioned Extract |
Generates a list of all Queries in a Study with status details and operational information according to the selected version. |
Event Progress Listing
The Event Progress Listing job generates a list of all Events in the Study, with status details and operational data, as a CSV file or report. The Event Progress Listing job is versionless.
Job Options
The following configuration options are available for the Event Progress Listing job:
-
Include Review Data: Users with the View SDV or View DMR permissions can choose to include SDV/DMR data in the listing.
-
Include Restricted Data: When this checkbox is selected, Vault includes events from restricted Forms if the job is initiated by a user with the Restricted Data Access permission. An event becomes Restricted when the data that triggers a dynamic event contains a Restricted form and is updated by a user without Restricted permission and the Restricted form is included in the submitted event. A user with Restricted permission must perform an action to clear the newly unneeded form.
Output
The Event Progress Listing job CSV export and report include the columns listed below. If your Study is using Data Model V1, the Event Signature Date, Freeze Date, and Lock Date columns will be blank in the CSV and report output. If your Study contains log events, the Event Date and Visit Method Frozen, Locked, and Signed columns will be blank.
| Column Header | Description | Notes |
|---|---|---|
| Study | Study Name | |
| Country | Study Country Name | |
| Site | Site number | |
| Subject | Subject number | |
| Subject Status | Current Subject status | |
| Event Group Label | Event Group Label | |
| Event Label | Event Label | |
| Event Group Sequence Number | Event Group Sequence Number | |
| Event Type | Event Type | |
| Event Date | Event Date entered | This column will be blank for log events. |
| Visit Method | Visit Method selected | |
| Event Status | Event status (Planned, Blank, In Progress, Submitted, Did Not Occur) | |
| Event Did Not Occur | Indicates if the event was marked as Did Not Occur (Yes/No value) | |
| Event Did Not Occur Reason | Change Reason entered if the event was marked as Did Not Occur | |
| Restricted Event | Shows if the event is restricted or not (Yes/No value) | |
| Early Planned Date | The low date relative to study design plan and not considered out of window | |
| Planned Date | The date from study design the event was planned for | |
| Late Planned Date | The high date relative to study design plan and not considered out of window | |
| Event Out of Window | If the event is out of window (below Early, above Late), or if missing (and past late grace) | This column will be blank for log events. |
| Days Out of Window | If Out of Window, display the number of days out of window | This column will be blank for log events. |
| SDV Plan | The SDV plan assigned to subject at time of generation | |
| Event SDV Required | Shows if SDV was required for the Event (Required, Optional, Not Required, Blank) | Review Rollup V2: The value (Yes/No) for this column will roll up from forms at the log event when the Create Event Summaries for Log Events feature is enabled. This behavior affects the event_summary__v.sdv_is_required__v field. This column will be blank for studies using Review Rollup V1. |
| Event Requires Re-SDV | Shows if SDV is required again for the Event (Yes/No value). This column will be blank for studies created prior to the 23R3 release. | |
| Event SDV Complete | Shows whether the Event completed SDV (Yes/No value) | Review Rollup V2: The value (Yes/No) for this column will roll up from forms at the log event when the Create Event Summaries for Log Events feature is enabled. This behavior affects the event_summary__v.sdv_completed__v field. This column will be blank for studies using Review Rollup V1. |
| % Event SDV Complete | Shows the percentage of SDV completion for the Event | |
| Event First SDV Completion Date | Shows the datetime when everything required for SDV was first marked as SDV Complete for this Event | Review Rollup V2: The value (date) for this column will roll up from forms at the log event when the Create Event Summaries for Log Events feature is enabled. This behavior affects the event_summary__v.first_sdv_date__v field. This column will be blank for studies using Review Rollup V1. |
| Event SDV Completion Date | Shows datetime when everything required for SDV was marked as SDV Complete for this Event | Review Rollup V2: The value (date) for this column will roll up from forms at the log event when the Create Event Summaries for Log Events feature is enabled. This behavior affects the event_summary__v.sdv_completed_date__v field. This column will be blank for studies using Review Rollup V1. |
| Event Date SDV Required | Shows whether the Event Date is required for SDV (Required, Optional, Not Required, Blank value) | |
| Event Date SDV Complete | Shows whether the Event Date underwent SDV (Yes/No value) | |
| Event Date First SDV Completion Date | Shows the date that SDV for all forms in the event, and for Event Date and Visit Method if required, was first completed. This value will be cleared if the form or event is reset. | |
| Event Date SDV Completion Date | Shows the date that SDV was completed for the Event Date. | |
| Visit Method SDV Required | Shows if SDV was required for the Visit Method (Required, Optional, Not Required, Blank) | |
| Visit Method SDV Complete | Shows whether the Visit Method completed SDV (Yes/No value) | |
| Visit Method SDV Completion Date | Shows the date when the Visit Method underwent SDV | |
| Total Forms SDV Required | Total number of forms completed for this event, where at least one field is SDV required | |
| Submitted Forms SDV Required | Number of submitted forms where SDV is required at the event | |
| Total Forms SDV Complete | Shows the total number of forms that completed SDV | |
| % Forms SDV Complete | Percentage of forms that underwent SDV at this event | |
| Forms SDV Completion Date | The date on which all forms at this event completed SDV for this subject | |
| DMR Plan | The DMR plan assigned to subject at time of generation | |
| Event DMR Required | Shows if DMR was required for the Event (Required, Optional, Not Required, Blank) | Review Rollup V2: The value (Yes/No) for this column will roll up from forms at the log event when the Create Event Summaries for Log Events feature is enabled. This behavior affects the event_summary__v.dmr_is_required__v field. This column will be blank for studies using Review Rollup V1. |
| Event Requires Re-DMR | Shows if DMR is required again for the Event (Yes/No value) | |
| Event DMR Complete | Shows whether the Event completed DMR (Yes/No value) | Review Rollup V2: The value (Yes/No) for this column will roll up from forms at the log event when the Create Event Summaries for Log Events feature is enabled. This behavior affects the event_summary__v.dmr_completed__v field. This column will be blank for studies using Review Rollup V1. |
| % Event DMR Complete | Shows the percentage of DMR completion for the Event | |
| Event First DMR Completion Date | Shows the date that DMR for all forms in the Event, and for Event Date and Visit Method if required, was first completed. This value will be cleared if the form or event is reset. | Review Rollup V2: The value (date) for this column will roll up from forms at the log event when the Create Event Summaries for Log Events feature is enabled. This behavior affects the event_summary__v.first_dmr_date__v field. This column will be blank for studies using Review Rollup V1. |
| Event DMR Completion Date | Shows the datetime when everything required for DMR was marked as DMR Complete for this Event | Review Rollup V2: The value (date) for this column will roll up from forms at the log event when the Create Event Summaries for Log Events feature is enabled. This behavior affects the event_summary__v.dmr_completed_date__v field. This column will be blank for studies using Review Rollup V1. |
| Event Date DMR Required | Shows whether the Event Date is required to complete DMR (Required, Optional, Not Required, Blank value) | |
| Event Date DMR Complete | Shows whether the Event Date completed DMR (Yes/No value) | |
| Event Date First DMR Completion Date | The date when the Event Date was first marked as DMR Complete | |
| Event Date DMR Completion Date | The date when the Event Date completed DMR | |
| Visit Method DMR Required | Shows if DMR was required for the Visit Method (Required, Optional, Not Required, Blank) | |
| Visit Method DMR Complete | Shows whether the Visit Method completed DMR (Yes/No value) | |
| Visit Method DMR Completion Date | Shows the date when the Visit Method underwent DMR | |
| Total Forms DMR Required | Total number of forms completed for this Event, where at least one field is DMR required | |
| Submitted Forms DMR Required | Number of submitted forms where DMR is required at the Event | |
| Total Forms DMR Complete | Shows the total number of forms that completed DMR | |
| % Forms DMR Complete | Percentage of forms that underwent DMR at this Event | |
| Forms DMR Completion Date | The date on which all forms at this Event completed DMR for this subject | |
| Event Date Frozen | Shows if the Event Date was frozen (Yes/No value) | |
| Event Date Frozen Date | The date that the Event Date was frozen | |
| Event Date Locked | Shows if the Event Date was locked (Yes/No value) | |
| Event Date Locked Date | The date that the Event Date was locked | |
| Event Signed | Shows if event is signed (Yes/No value) | The value (Yes/No) for this column will roll up from forms at the log event when the Create Event Summaries for Log Events feature is enabled. This behavior affects the event_summary__v.event_signed__v field for studies using Data Model 2 and the event__v.rev_sign__v field for studies using Data Model 1. |
| Event Date Signature Date | The date when the event was signed | The value (date) for this column will roll up from forms at the log event when the Create Event Summaries for Log Events feature is enabled. This behavior affects the event_summary__v.event_signed_date__v field for studies using Data Model 2. This column will be blank for studies using Data Model 1. |
| Visit Method Frozen | Shows if the Visit Method was frozen (Yes/No value) | This column will be blank for log events |
| Visit Method Frozen Date | The date that the Visit Method was frozen | |
| Visit Method Locked | Shows if the Visit Method was locked (Yes/No value) | This column will be blank for log events |
| Visit Method Locked Date | The date that the Visit Method was locked | |
| Visit Method Signed | Shows if the Visit Method was signed (Yes/No value) | This column will be blank for log events |
| Visit Method Signature Date | The date that the Visit Method was signed | |
| Event Frozen | Shows if the Event is frozen (Yes/No value) | The value (Yes/No) for this column will roll up from forms at the log event when the Create Event Summaries for Log Events feature is enabled. This behavior affects the event_summary__v.event_frozen__v field for studies using Data Model 2 and the event__v.rev_frozen__v field for studies using Data Model 1. |
| Event Frozen Date | The date when the Event was frozen | The value (date) for this column will roll up from forms at the log event when the Create Event Summaries for Log Events feature is enabled. This behavior affects the event_summary__v.event_frozen_date__v field for studies using Data Model 2. This column will be blank for studies using Data Model 1. |
| Event Locked | Shows if the Event is locked (Yes/No value) | The value (Yes/No) for this column will roll up from forms at the log event when the Create Event Summaries for Log Events feature is enabled. This behavior affects the event_summary__v.event_locked_date__v field for studies using Data Model 2 and the event__v.rev_locked__v field for studies using Data Model 1. |
| Event Locked Date | The date when the Event was locked | The value (date) for this column will roll up from forms at the log event when the Create Event Summaries for Log Events feature is enabled. This behavior affects the event_summary__v.event_frozen_date__v field for studies using Data Model 2. This column will be blank for studies using Data Model 1. |
| Event ID | Vault ID of the event | |
| Last Run of Listing | Datetime when the listing was last run |
Form Progress Listing
The Form Progress Listing job generates a list of all Forms in the Study, with status details and operational data, as a CSV file. The Form Progress Listing job is versionless.
Job Options
The following configuration options are available for the Form Progress Listing job:
-
Include Restricted Data: When this checkbox is selected, Vault includes data from restricted Forms if the job is initiated by a user with the Restricted Data Access permission.
-
Include Item Counts: When this checkbox is selected, Vault includes item counts for All Items, Items with Values, Items with Value Change, Items SDV Required Completed, Items SDV Required, Items DMR Required Completed, and Items DMR Required.
Output
The Form Progress Listing job CSV export and report include the columns listed below. If your Study is using Data Model V1, the SDV Required, Items SDV Required Completed, Items SDV Required, SDV %, SDV Age, DMR Required, Items DMR Required Completed, Items DMR Required, DMR %, and DMR Age columns will be blank in the job export and report output.
Requires Re-SDV and Requires Re-DMR columns in the Form Progress Listing: For studies created after 23R3, ‘Requires Re-SDV’ and ‘Requires Re-DMR’ are recorded in the Form Progress Listing. These columns will be blank for studies created before 23R3 or for studies where the First Review Date is not enabled.
| Column Header | Description |
|---|---|
| Study | Study Name |
| Country | Study Country Name |
| Site | Site Number |
| Subject | Subject Number |
| Subject Status | Current Subject Status |
| Event Group Label | Event Group Label of the Event Group where the Form is |
| Event Label | Event Label of the Event where the Query is |
| Event Group Sequence Number | Event Group Sequence Number |
| Event Date | Event Date entered |
| Form Label | Form Label |
| Form Sequence Number | Form Sequence Number |
| Form Status | Form Status (Planned, Blank, In Progress, Submitted, In Progress Post Submit) |
| First Submission Date | Datetime when the Form was first submitted |
| Latest Submission Date | Datetime when the Form was last submitted |
| Number of Submits | Total number of Form submissions |
| Restricted Form | Shows if the Form is Restricted or not (Yes/No value) |
| Complete | Shows if the Form is complete/submitted, including ILB forms (Yes/No value) |
| Intentionally Left Blank | Shows if the Form was Intentionally Left Blank (Yes/No value) |
| Intentionally Left Blank Reason | The reason the Form was marked as Intentionally Left Blank |
| Marked for Removal | Shows whether the Form is no longer needed |
| Late | Shows if the Form is late based on the Event Date and the number of overdue days allowed (Yes/No value) |
| Days Overdue | Shows the number of days beyond the overdue date |
| All Items | Calculates the total number of items on the form |
| Items with Values | Calculates the number of items on the form with a saved value |
| Items with Value Change | Calculates the number of items on the form with at least one data value change |
| SDV Plan | SDV Review Plan assigned to the Subject |
| Form SDV Override Plan | SDV Review Plan override for the Form (from an override rule) |
| Form SDV Required | Shows whether SDV is required for the Form |
| Form Requires Re-SDV | Shows whether SDV needs to be completed again after being cleared due to updates by the site |
| Form SDV Complete | Shows whether SDV was completed for the Form |
| Items SDV Required | Number of items on the Form where SDV is required |
| Items SDV Required Completed | Number of items on the Form where SDV is required and completed |
| Form SDV % | Percentage of required SDV complete for the Form |
| Form SDV Age | How long the Form has been submitted without SDV |
| Form First SDV Completion Date | Date that SDV was first completed for the Form |
| Form SDV Completion Date | Date that SDV was completed for the Form |
| DMR Plan | DMR Review Plan assigned to the Subject |
| Form DMR Override Plan | DMR Review Plan override for the Form (from an override rule) |
| Form DMR Required | Shows whether DMR is required for the Form |
| Form Requires Re-DMR | Shows whether DMR needs to be completed again after being cleared due to updates by the site |
| Form DMR Complete | Shows whether DMR was completed for the Form |
| Items DMR Required | Number of items on the Form where DMR is required |
| Items DMR Required Completed | Number of items on the Form where DMR is required and completed |
| Form DMR % | Percentage of DMR complete for the Form |
| Form DMR Age | How long the Form has been submitted without DMR |
| Form First DMR Completion Date | Date that DMR was first completed for the Form |
| Form DMR Completion Date | Date that DMR was completed for the Form |
| Frozen | Whether or not the Form is frozen (Yes/No value) |
| Freeze Date | Date the Form was frozen |
| Locked | Whether or not the Form is locked (Yes/No value) |
| Lock Date | Date the Form was locked |
| Signed | Whether or not the Form is signed |
| Sign Date | Date the Form was signed |
| Last Signed Date | Date the Form was last signed |
| Total Queries | Total number of queries on the Form |
| Open Queries | Total number of open queries on the Form |
| Answered Queries | Total number of answered queries on the Form |
| Closed Queries | Total number of closed queries on the Form |
| Form Vault ID | Vault ID of the Form |
| Last Run of Listing | Datetime when the listing was last run |
Query Detail Listing
The Query Detail Listing job generates a list of all Queries in the Study, with status details and operational data, as a CSV file. Users can filter this listing by Query Status, Query Team, Site, and Form. The output only includes Queries from restricted Forms if the user selected the Include Restricted Data checkbox and has the Restricted Data Access permission. The Query Progress Listing job is versionless. Note that if you have the Show Queries in the User Language setting configured, queries will display in the user’s language, not the vault language.
Enabling Team Query Restrictions and the Query Detail Listing: When the Team Query Restrictions study setting is not enabled, the Role column in the Query Detail Listing is populated with the user’s current role at the time of job generation. This information is not recorded in the query record. When the Team Query Restrictions setting is enabled in an ongoing study, the user’s role and team are stored on the query record at the time of the query action for queries moving forward and will populate in the Roles and Teams columns in the Query Detail Listing. Queries that were created prior to the Team Query Restrictions setting being enabled are not assigned a query team. If you want to keep the current roles for existing queries in the Query Detail Listing, we do not recommend that you enable Query Teams in an ongoing study.
Job Options
The following configuration options are available for the Query Detail Listing job:
- Include Restricted Data: When this checkbox is selected, Vault includes queries from restricted Forms if the job is initiated by a user with the Restricted Data Access permission.
Output
The Query Detail Listing job CSV export and report include the columns listed below. If your Study isn’t using Query Teams, the Query Team, Created by Query Team, Answered by Query Team, Closed by Query Team, Created by Role, Answered by Role, and Closed by Role columns will be blank in the CSV and report output.
| Column Header | Description |
|---|---|
| Study | Study Name |
| Country | Study Country Name |
| Site | Site Number |
| Subject | Subject Number |
| Subject Status | Current Subject Status |
| Event Group Label | Event Group Label of the Event Group where the query is |
| Event Label | Event Label of the Event where the query is |
| Event Group Sequence Number | Event Group Sequence Number |
| Event Date | Event Date entered |
| Visit Method | Visit method selected |
| Form Label | Form Label of the Form where the query is |
| Form Sequence Number | Form Sequence Number |
| Form Status | Form Status |
| Item Group Label | Item Group Label of the Item Group where the query is |
| Item Group Sequence Number | Item Group Sequence Number |
| Item OID (External ID) | External ID (OID) of the item that the query is attached to |
| Item Label | Label of the item that the query is attached to |
| Query ID | The query name (VV-XXXXX) (not the internal vault ID) |
| Query Status | Current Status of the query |
| Restricted Query | Shows if the query is Restricted or not (Yes/No value) |
| Query Team | Query Team assigned to the query |
| Observed Source Value | Shows the observed source value for studies using Quick Queries |
| Value Confirmed | Shows whether the observed source value was confirmed for studies using Quick Queries (Yes/No value) |
| Source Type | Source type of the query |
| Source System Name | Value optionally set by an API query addition, describing the name of the query source system |
| Source User | Value optionally set by an API query addition, describing the user who opened the query |
| Source ID | Unique identifier from the source of the query |
| Days Unresolved | Days the query has been unresolved |
| Manual Query | Shows if the query was manually created (Yes/No value) |
| Query Rule | If a query rule, the rule that originates the query |
| Original Query Text | First message text of the query |
| Original Query Text in User's Language | Original text of the query in the User’s language |
| Original Query Text in English | Original text of the query in English |
| Latest Query Comment | Latest query message |
| Latest Query Answer Text | Last answer of the query |
| Number of Query Messages | The number of query messages on a query |
| Item Value Before Query | Value of the item the query is attached to before query creation |
| Item Value Now | Value of the item the query is attached to at the time of run |
| Item Value Changed | Shows whether the value of the queried item changed (Yes/No value) |
| Query Caused Data Change | Shows whether the data changed after query creation (Yes/No value). This field is determined by checking if the item value was modified between creation and closing of the query (by dates). |
| Query Created Date | Datetime of the creation of the query |
| Query Created By | User that created the query (or System) |
| Created By Role | Role name of the user that created the query (or System) |
| Created by Query Team | Query Team of the user that created the query |
| Query Answered Date | Datetime of last answer of the query |
| Query Answered By | User that last answered the query |
| Answered by Role | Role name of the user that last answered the query |
| Answered by Query Team | Query Team of the user that last answered the query |
| Query Closed Date | Datetime of last closing of the query |
| Query Closed By | User that last closed the query (or System) |
| Closed By Role | Role name of the user that last closed the query (or System) |
| Closed by Query Team | Query Team of the user that last closed the query |
| Query Vault ID | Vault Query Object |
| Last Run of Listing | Datetime when the listing was last run |
Subject Progress Listing
The Subject Progress Listing job generates a list of all Subjects in the Study, with status details and operational data, as a CSV file. The Subject Progress Listing job is versionless.
Job Options
The following configuration options are available for the Subject Progress Listing job:
- Include Restricted Data: When this checkbox is selected, Vault includes data from restricted Forms if the job is initiated by a user with the Restricted Data Access permission.
Output
The Subject Progress Listing job CSV export and report include the following columns:
| Column Header | Description |
|---|---|
| Study | Study Name |
| Country | Study Country Name |
| Site | Site Number |
| Subject | Subject Number |
| Subject Status | Current Subject status |
| Restricted Subject | Shows if the Subject is Restricted (Yes/No value) |
| Most Recent Visit | Event Label of the last visit/event |
| Date of Most Recent Visit | Event Date of the last visit/event |
| Method of Most Recent Visit | Visit Method of the last visit/event |
| Next Event | Event Label of the next scheduled visit/event |
| Entry Complete | Shows if all forms expected for a Subject have been submitted (Yes/No value) |
| SDV Plan | SDV Plan assigned to the Subject |
| Subject SDV Required | Shows whether SDV is required for the Subject |
| Subject SDV Complete | Shows if all forms and all event dates that require SDV have been marked SDV for the Subject |
| Subject SDV Completion Date | The date that SDV was completed for the Subject |
| Forms SDV Completion Date | The date that SDV was completed on all forms for the Subject |
| DMR Plan | DMR plan assigned to the Subject |
| Subject DMR Required | Shows whether DMR is required for the Subject |
| Subject DMR Complete | Shows if all forms and all event dates that require DMR have been marked DMR for the Subject |
| Subject DMR Completion Date | The date that DMR was completed for the Subject |
| Forms DMR Completion Date | The date that DMR was completed on all forms for the Subject |
| Frozen | Shows if all forms, event dates, and visit methods have been frozen for the Subject |
| Locked | Shows if all forms, event dates, and visit methods have been locked for the Subject |
| Signed | Shows if all forms, event dates, and visit methods have been signed for the Subject |
| All Queries Closed | Shows if all queries for the Subject have been closed |
| Clean | Shows if a Subject is "clean," meaning that all forms, event dates, and visit methods are submitted, signed, and locked and all queries are closed. SDV and DMR should also be completed (Yes/No value) |
| Forms | Total number of forms for Subject |
| Forms Complete | Number of forms submitted |
| Forms Incomplete | Number of forms that are blank or in progress (e.g. forms that are not submitted yet) |
| Forms SDV Required | Number of forms that are SDV required for the subject |
| Submitted Forms SDV Required | Number of submitted forms that are SDV required for the subject |
| Forms SDV Complete | Number of forms that were marked as SDV reviewed for the subject |
| % Forms SDV Complete | Percentage of forms that were marked as SDV reviewed for the subject compared to the number of forms marked SDV required |
| Forms Not SDV | Number of forms submitted that require SDV based on the review plan but have not been marked for SDV |
| Event Dates Not SDV | Number of event dates that require SDV that have not been marked SDV for the Subject |
| Visit Methods Not SDV | Number of visit methods that require SDV that have not been marked SDV for the Subject |
| Forms DMR Required | Number of forms that are DMR required for the subject |
| Forms DMR Complete | Number of forms that were marked as DMR reviewed for the Subject |
| Submitted Forms DMR Required | Number of submitted forms that are DMR required for the subject |
| % Forms DMR Complete | Percentage of forms that were marked as DMR reviewed for the subject compared to the number of forms marked DMR required |
| Forms Not DMR | Number of forms submitted that require DMR based on the review plan but have not been marked for DMR |
| Event Dates Not DMR | Number of event dates that require DMR that have not been marked DMR for the Subject |
| Visit Methods Not DMR | Number of visit methods that require DMR that have not been marked DMR for the Subject |
| Forms Not Frozen | Total number of submitted forms that are not frozen |
| Event Dates Not Frozen | Total number of event dates that are not frozen |
| Visit Methods Not Frozen | Total number of visit methods that are not frozen |
| Forms Not Locked | Total number of submitted forms that are not locked |
| Event Dates Not Locked | Total number of event dates that are not locked |
| Visit Methods Not Locked | Total number of visit methods that are not locked |
| Forms Not Signed | Total number of submitted forms that are not signed |
| Event Dates Not Signed | Total number of event dates that are not signed |
| Visit Methods Not Signed | Total number of event dates that are not signed |
| Total MC | Total Medical Coding Requests for the Subject |
| MedDRA MC | Total MedDRA Medical Coding Requests |
| WHODrug MC | Total WHODrug Medical Coding Requests |
| MedDRA MC Need Coding | MedDRA Medical Coding Requests that are not yet coded |
| WHODrug MC Need Coding | WHODrug Medical Coding Requests that are not yet coded |
| Total Queries | Total number of queries |
| Open Queries | Total number of open queries |
| Answered Queries | Total number of answered queries |
| Closed Queries | Total number of closed queries |
| Subject Vault ID | Vof ID of the Subject |
| Last Run of Listing | Datetime when the listing was last run |
Note that Required columns will appear before Completed columns in the listing jobs output.
Scheduling Study Listing Report Jobs
The Study Listing reports available in the Reports tab are based on Study data up to the last time the Study Listing job was run. Study Listing Reports can only be created by scheduling recurring Study Listing jobs. We recommend that you schedule Study Listings as daily jobs to ensure that the data provided by the Study Listing report in the Reports tab is up to date to within the past 24 hours. You can check the last date and time the Study Listing job was run in the Last Run of Listing column of the report. Learn more about running ad hoc and scheduled jobs in EDC Tools.
To schedule a Study Listing Report Job:
- Navigate to Tools > EDC Tools > Job Schedule for your Study.
- Select a Study Listing job from the Type dropdown menu.
- Different options display depending on the type of job you selected. Select the required options. We recommend selecting Include Restricted Data.
-
Select a Frequency. You can only create Study Listing Reports from recurring Study Listing jobs. We recommend scheduling your Study Listing job to run daily.
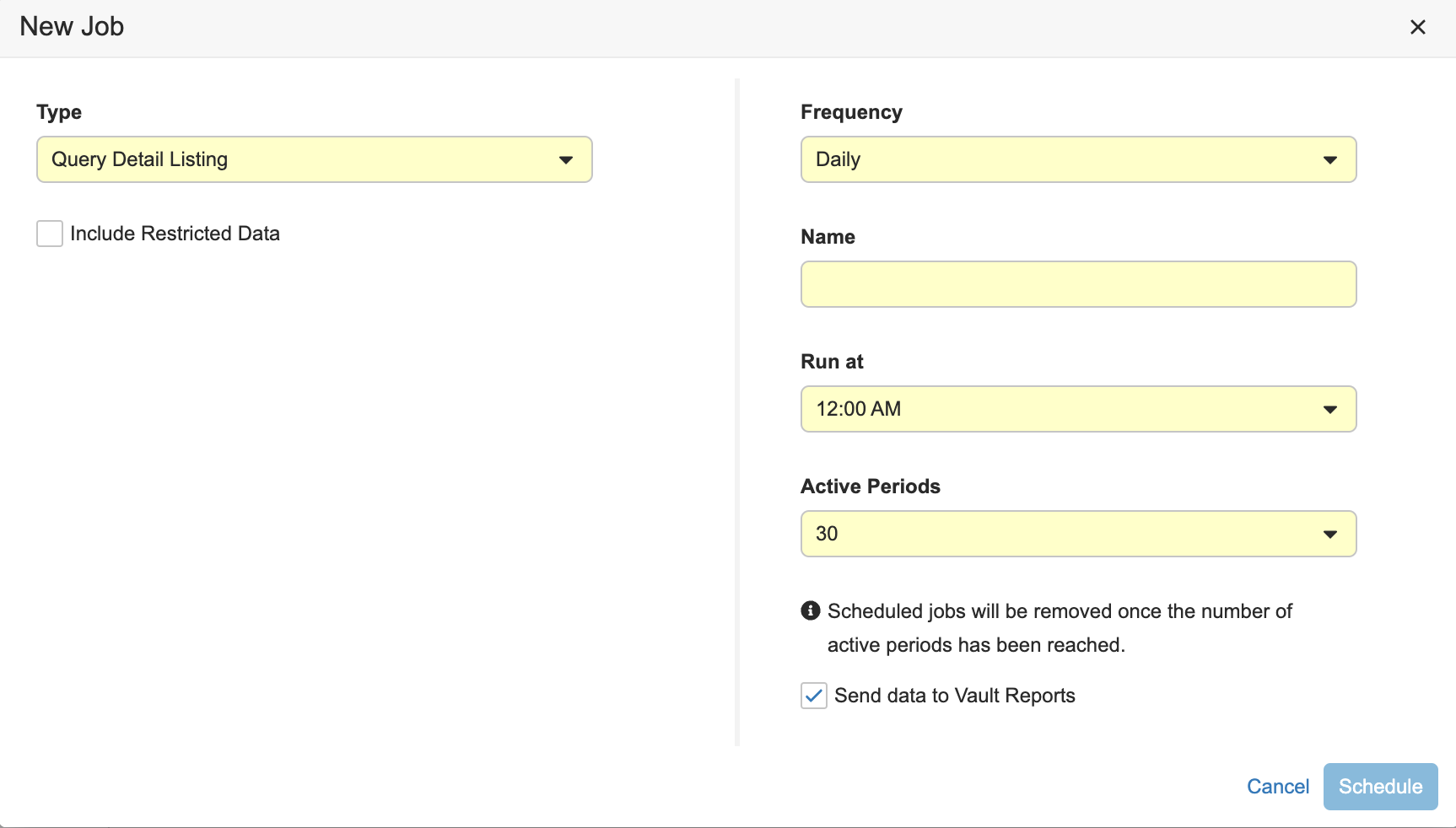
- Enter a Name for your job.
- Select a time for your report to run from the Run at dropdown.
- Select Send data to Vault Reports.
- Click Schedule. Vault runs scheduled jobs at the configured time, and you will receive an email notification with a link to download the job log and relevant output files each time the job runs.
Job Frequency: To prevent slowdown of your vault, we recommend that you schedule Study Listing jobs to create reports during off-peak hours.
Once the Study Listing job has run, populating data to the Reports tab, users can customize the report as needed. Learn more about how to create customized versions of standard report templates.
Sharing Study Listing Reports
To make Study Listing report templates viewable by users, you must share them with the users or user groups. User access to report data is automatically restricted by the Study, Site, and restricted data access permissions. Learn more about how to share reports.
Study Listing Templates: The Vault Owner must share Study Listing templates with the Data Management or Clinical Teams to make them accessible.