Providing Signatures
You can sign and date in EDC using eSignature. Veeva EDC eSignature meets the requirements of FDA 21 CFR Part 11. You can sign at the Form, Event, or Casebook (Subject) level and the signature will apply to all eligible forms and event dates. Some actions can break a signature after it is applied. When this happens, EDC invalidates your signature and requires you to sign again. Learn more about actions that break a signature.
If your study is using Data Model 2 and the Enable Bulk Casebook Signature feature is configured, you can bulk sign all eligible Subjects at a Site. Contact Veeva Support to enable this feature then configure at the Study level in EDC Tools > Study Settings.
Prerequisites
Before you can provide a signature in your Study, a study designer must first create a casebook-type Signature Definition in Studio.
Users with the standard CDMS Principal Investigator application role can perform the actions described above by default. If your vault uses custom Application Roles, your role must have the following permissions:
| Type | Permission Label | Controls |
|---|---|---|
| Standard Tab | Data Entry Tab | Ability to access the Data Entry tab |
| Functional Permission | Sign | Ability to provide an electronic signature on study data |
If your Study contains restricted data, you must have the Restricted Data Access permission to view it.
Learn more about Study Roles.
Vault supports authenticating with a Single Sign-on (SSO) provider when performing the following actions:
Data Entry:
- Signing at the Form or Casebook level
- Accepting Study closeout PDFs
Randomization:
- Emergency Unmasking
- Reveal treatment
- Redispense Kit/Device
- Verify list
Labs:
- Updating Normal Ranges that are In Use
When Can You Sign?
You can sign a Form or Event when the following conditions are met:
- Any Forms are Submitted
- The Form, Event, and Casebook are unlocked
- There are no open queries (ability to sign depends on the signature level)
When there is an open query on an Event:
- You can’t sign Event Dates or Visit Methods on the Event.
- You can sign Forms within that Event.
- You can sign at the Casebook level, but Vault only applies your signature to Event Dates with no open queries. Vault then applies your signature to all Forms, including those in the Event.
When there is an open query on an Item, but there is no open query on the Event Date:
- You can’t sign a Form with an open query.
- You can sign at the Event level, but Vault only applies your signature to Forms with no open queries.
If your study is using Data Model 2 and the Enable Sign with Open Queries feature is configured, you can sign Forms and Event Dates with open queries. Contact Veeva Support to enable this feature then configure at the Study level in EDC Tools > Study Settings.
How to Sign
You sign by entering your login credentials (username and password) for the vault. Note that Vault prevents you from using saved passwords for compliance reasons. Even if you’ve saved your password in your browser or a password manager, you’ll have to type the password to provide an eSignature.
The Sign button and Sign Form action only display for users that have permission to provide signatures.
To provide an eSignature:
- Click Sign:
- Select the events to sign.
- Enter your login credentials in the Apply Electronic Signature dialog.
- Click Complete.
Bulk Casebook Signature
If your study is using Data Model 2 and the Enable Bulk Casebook Signature feature is configured, you can bulk sign all eligible Subjects at a Site. To bulk sign Casebooks, a Casebook-type signature definition must exist for your Study. Otherwise, this action is not available.
Bulk Casebook Signature Enablement: If your vault was created before the 24R3 release (December 2024), contact Veeva Support to enable this feature. Then, a lead data manager can enable it in your Study from EDC Tools. If your vault was created after the 24R3 release, this feature can be enabled by a lead data manager in EDC Tools.
To bulk sign:
-
Click Sign Casebooks button in the top right hand corner of the page after navigating to your Subject list.
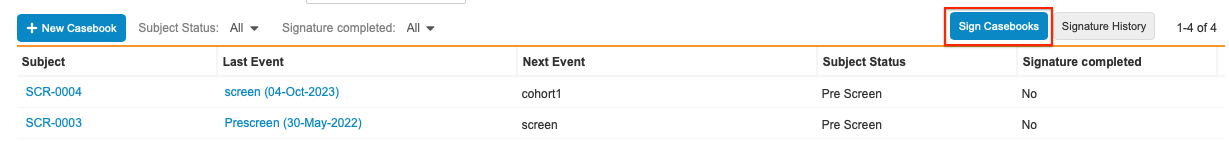
- Select where to apply the signature: All Eligible Subjects or Selected Subjects.
- If you chose Selected Subjects, select the checkbox next to each Subject you’d like to apply the signature.
- Click Sign Now.
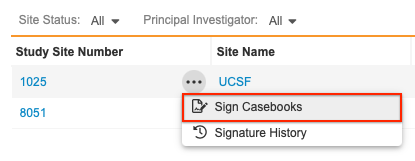
You can also bulk sign from the Site listing page by selecting Sign Casebooks from the Site’s Actions menu.
How to Clear a Signature
To clear an eSignature:
- Select the Clear Signature action:
- For a Form, open the Form and click on Clear Signature.
- For a subject Casebook click Clear Casebook Signature from the casebook schedule.
- For an Event, click Clear Event Signature from the Form’s Actions Menu.
- In the Clear Signature dialog, click Clear Signature.
The following data changes will automatically clear a signature:
- Editing form data
- Adding a repeating Item Group
Actions that Break a Signature
Some actions in the Data Entry tab can break the Signature on a Form, Casebook, or Event.
Actions in Data Entry that can break a signature include:
- Editing a Form, which involves changes to Item data (breaks signature for the Form)
- Resetting a Form (breaks signature for the Form)
- Resetting an Event (breaks signature for the Forms in the Event)
- Editing an Event Date (breaks signature for the Event Date)
- Editing a Visit Method (breaks signature for the Visit Method)
A retrospective amendment is a change to the study protocol that alters the study design. Retrospective amendments that involve changes to certain study objects can also break a signature. Learn more about Retrospective Amendments.
Changes to objects via retrospective amendment that can break a signature include:
- Changes to codelist labels (only breaks the signature if the label changed is the selected label) (breaks signature for the Form)
- Changes to a different Form linked to a Form Link Item (breaks signature for the Form containing the Form Link Item)
- Addition of new Items to a Form (breaks signature for the Form)
- Creation of a new Lab panel (breaks signature for the Form)
- Addition of a new Analyte (breaks signature for the Form)
- Addition of new Forms to the schedule (breaks signature for Event Date and Casebook)