About EDC Clinical Reporting
Clinical Reporting provides reporting access to EDC clinical data, captured in the Vault EDC application.
Clinical Reporting can be accessed from Vault CDMS via the Clinical Reporting tab.
Listings
Data is available automatically from each EDC form via Core Listings. Each Core Listing displays certain metadata about the Study (this can be configured by an administrator) and each data collection Item on the Form.
Data managers can combine data across Forms via Custom Listings, which they can build with a drag and drop listing builder interface. Custom listings can combine or compare data across multiple Forms with comparison filters. Users can also copy listings from other Studies in the same vault.
All listings provide indicators and messages for outstanding queries with the ability to navigate directly to an Item in the EDC Review UI to action queries. Data managers can sort and filter these listings. They can also download listing data to a CSV file.
Queries
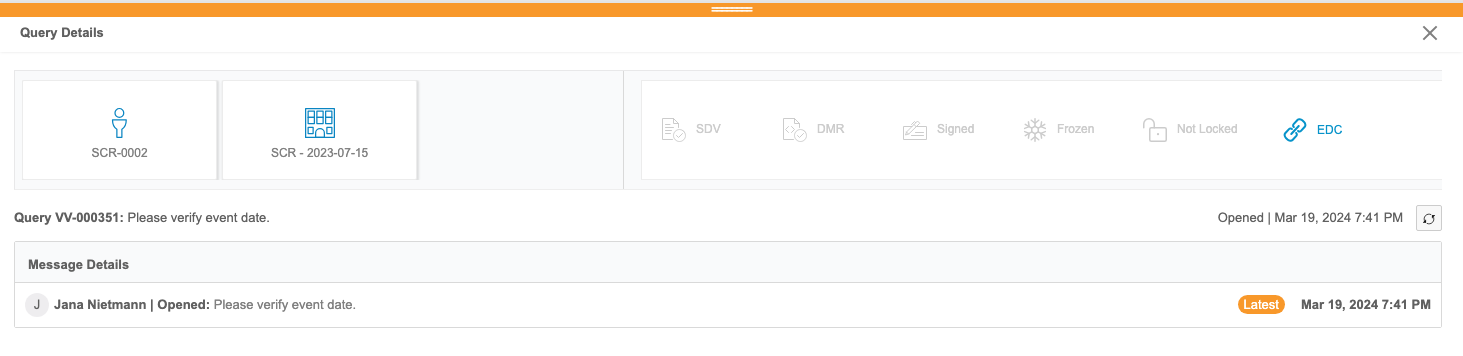
Clinical Reporting users can review listings of queries from Vault EDC. These reports aren’t customizable. Users can view details about each query, including all of its comments and a link to view the query in EDC from the Query Details panel.
Users can also review queries in the context of their forms from any core or custom listing in Clinical Reporting via the Cell Details panel.
Exports
Data managers can group data listings together for export. For each Export Definition, they can add one or more listings from the Study. Users can generate export packages from a definition as CSV or SAS (sas7bdat) files. They can then download the package directly or send it to an external, FTP destination. Data managers can also schedule recurring exports.
There are three (3) types of exports:
- None
- Raw
- SDTM
Import
Clinical Reporting automatically imports data from Vault EDC every fifteen (15) minutes, with no action required on behalf of the user. Clinical Reporting provides the ability to monitor this process from within the application. Clinical Reporting is updated with new study design changes every day at 12:00 AM GMT.
EDC Clinical Reporting & CDB Workbench
The EDC Clinical Reporting application contains a limited set of functions from the Clinical DataBase (CDB) Workbench application. The list below describes the differences between these two applications:
- CDB Workbench allows for the ingestion of third party data (third party EDC vendors, safety data, RTSM, and more).
- CDB Workbench allows for access to Clinical Query Language (CQL), a Veeva proprietary scripting language. This allows for the creation of more complex custom listings.
- Views are available in CDB Workbench . Views support custom listings by combining multiple forms for programming reference.
- Checks are available in CDB Workbench . Checks create queries automatically when defined conditions are met.
- CDB Workbench allows access to Review Listings, which allow data reviewers to track review progress over time while retaining the review status.
- CDB Workbench includes a Dashboard and Clean Patient Tracker for data reviewers to visualize their data cleaning progress.
- CDB Workbench allows users to clone listings, checks, and views from one test study to another test study.
- CDB has a deployment workflow to move listings, checks, and views from a test environment into the production study environment.
If your organization is interested in upgrading to CDB Workbench , contact your Veeva Services representative for more information.
Returning to Other Applications
You can return to Vault CDMS or switch to Workbench from the Application Switcher (). When you select CDMS, Clinical Reporting returns you to the default tab in CDMS, typically the Review tab.