Protocol Deviations
Sponsors can track Protocol Deviations for a Study from Veeva Clinical Data, in both EDC and CDB. Protocol Deviations, or “PDs”, can be created in Workbench manually or programmatically, via Checks. Similarly, those PDs created in EDC are created manually by monitors or programmatically via Rules. This feature is designed to help streamline study oversight and enhance data quality by providing a centralized system for creating and managing protocol deviations across all your study data, including data from both EDC and external data sources.
For example, you may log a protocol deviation when third party lab data shows a Date of Collection prior to the EDC Informed Consent Date, or when a third party lab value indicates that the subject should be excluded from the study, but that isn’t captured in the I/E form in EDC.
You can create PDs as you review data in Workbench, as well as review those PDs created in EDC.
Prerequisites
Before you can work with protocol deviations in Workbench, a study designer must enable this feature and perform certain configuration tasks in Veeva EDC Studio.
The availability of this feature is controlled by the Enable Protocol Deviations setting in Studio > Settings.
A study designer must create Categories, Subcategories, and Severities for you to reference when creating Protocol Deviations. Learn more.
Users with the standard CDMS Data Manager and CDMS Lead Data Manager study roles can perform the actions described below by default. If your organization uses custom Study Roles, your role must grant the following permissions:
| Type | Permission Label | Controls |
|---|---|---|
| Standard Tab | Workbench Tab | Ability to access and use the Data Workbench application, via the Workbench tab |
| Functional Permission | View Protocol Deviations | Ability to view Protocol Deviations |
| Functional Permission | Edit Protocol Deviations | Ability to edit Protocol Deviations |
| Functional Permission | Create Protocol Deviations | Ability to create Protocol Deviations |
If your Study contains restricted data, you must have the Restricted Data Access permission to view it.
Learn more about Study Roles.
Categories, Subcategories & Severities
PDs are grouped into Categories, Subcategories, and Severities. These groupings are based on your organization’s requirements and provide additional information about your PDs.
Protocol Deviations from Veeva EDC
Workbench imports any Protocol Deviations created in Veeva EDC so that you may view deviations created in EDC and Workbench in a single location.
You can view PDs created in EDC from listings in Workbench, but you can’t edit them. The only action you can take against these PDs is to inactivate them.
Viewing Protocol Deviations
You can view PDs in your Study from either the Cell Details panel or from standard or custom protocol deviation listings.
Cell Details Panel
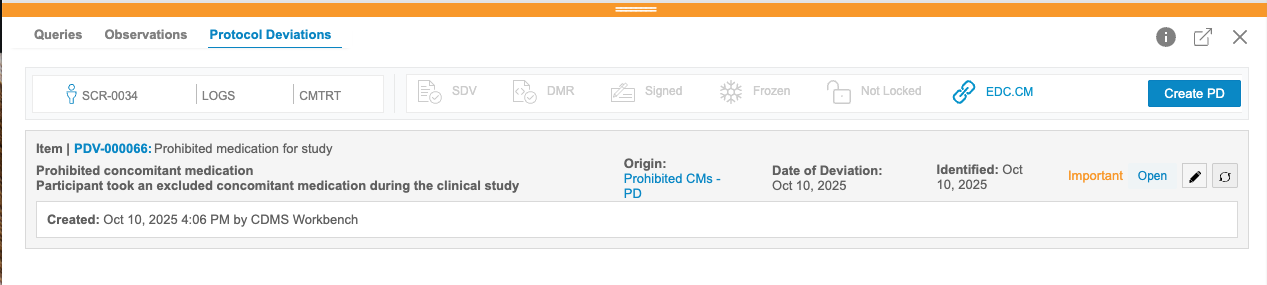
Workbench uses the PD cell decoration to indicate that there is a Protocol Deviation present on the cell. Click into a cell with the PD icon to the Cell Details panel. Then, click Protocol Deviations in the panel header to open that tab.
From here, you can review the details of the PD and inactivate it.
Protocol Deviation Listings
By default, Workbench includes a core PD listing for each Source in your Study. For example, if your study has two sources, EDC and Labs, Workbench generates a listing for each and labels it with the source name.
Workbench also includes the All Protocol Deviations listing, which includes all PDs from every source.
You can sort and filter these listings as needed.
Protocol deviation attributes are also available via CQL, so you can create custom listings to display PD information.
You can access these listings from the Protocol Deviations page. You can access the Protocol Deviations page from either the Study menu () on the Studies page or from the Navigation Drawer () after you’ve selected a Study.
|
|
Create a Protocol Deviation
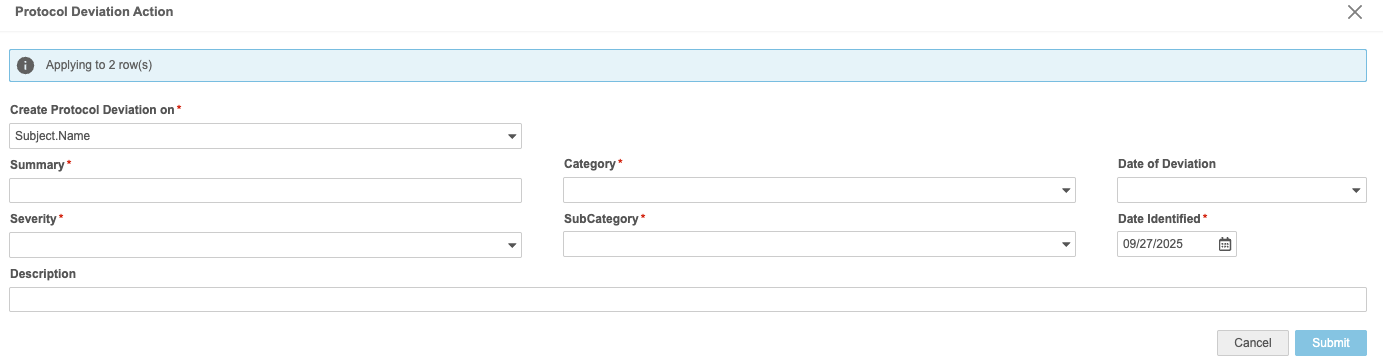
You can create a PD one at a time or you can create them in bulk using the same categorization.
You can create a PD at the Subject, Event, Form, or Item level.
- For a Subject-level PD, create the PD on the Subject.Name.
- For an Event-level PD, create the PD on the Event.Date.
- For a Form-level PD, create the PD on the Form.Name.
- For an Item-level PD, create the PD on the Item.
To create a single PD:
- Navigate to the listing in which you want to create a PD.
- Locate the Cell containing the data point you want to create a PD against.
- Click the Cell to open the Cell Details panel.
-
If needed, click Protocol Deviations to open that tab in the panel.
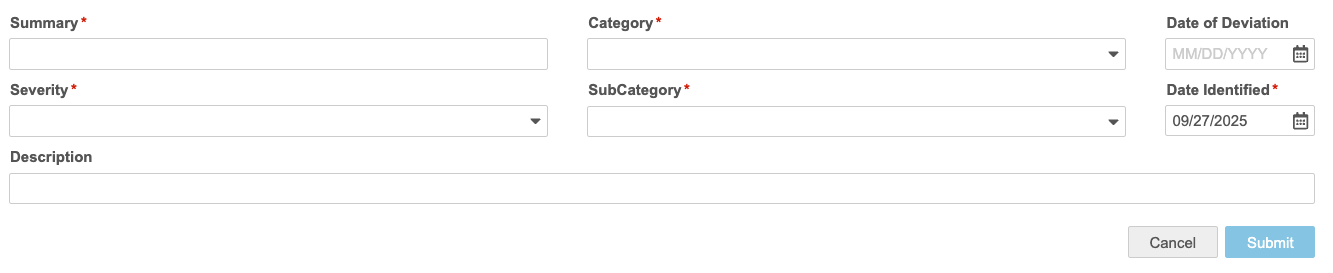
- Fill out the details of your PD:
- Summary: Provide a summary explanation of your PD.
- Category: Select the category for your PD.
- Date of Derivation: Enter or select the Date of Derivation. This is the date that the derivation occurred.
- Severity: Select the severity of the PD.
- SubCategory: Select the SubCategory of the PD.
- Date Identified: Enter or select the Date Identified. This is the date that the derivation was identified (this may occur at or after the Date of Derivation).
- Description (Optional): Enter a description of your PD.
- When finished, click Submit.
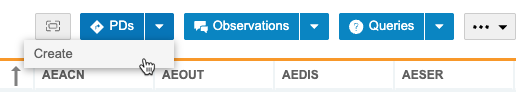
To create PDs in bulk:
- Navigate to the listing in which you want to create PDs.
-
Select the Rows (checkboxes) on which you want to create PDs.
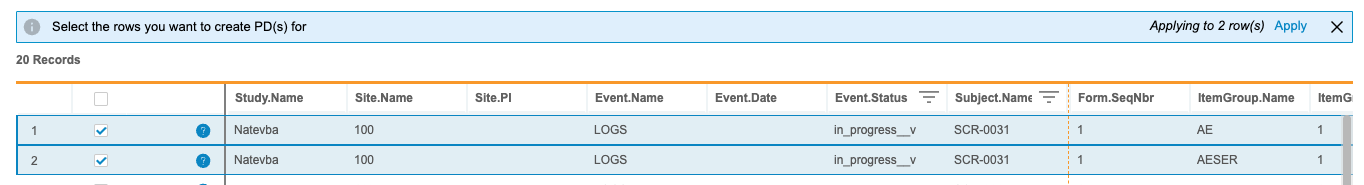
- Click Apply.
- In the Protocol Deviation Action panel, enter the details of your PD. This includes identifying which column to create the PD on.
- Create Protocol Deviation on: Select a column to create your PDs on.
- Summary: Provide a summary explanation of your PDs.
- Category: Select the category for your PDs.
- Date of Derivation: Enter or select the Date of Derivation. This is the date that the derivations occurred.
- Severity: Select the severity of the PDs.
- SubCategory: Select the SubCategory of the PDs.
- Date Identified: Enter or select the Date Identified. This is the date that the derivations were identified (this may occur at or after the Date of Derivation).
- Description (Optional): Enter a description of your PD.
- When finished, click Submit.
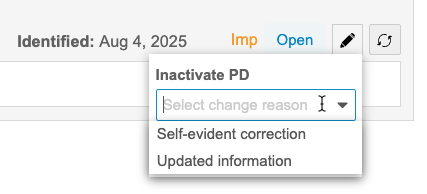
Inactivate a Protocol Deviation
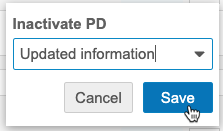
You can inactivate PDs. Inactivating a PD indicates that no further action is required or that it no longer applies. By default, there are two Change Reasons for inactivation: Updated information and Self-evident correction.
To inactivate a PD:
- Navigate to the PD that you want to inactivate.
- In the Protocol Deviation details, click Inactivate.
- Click Save.
Download Protocol Deviations as CSV
You can download your PDs as a CSV by downloading a protocol deviation listing. For other formats, you can create an Export Definition that contains the information you need.
To download your PD listing:
- Navigate to the protocol deviation listing that you want to download.
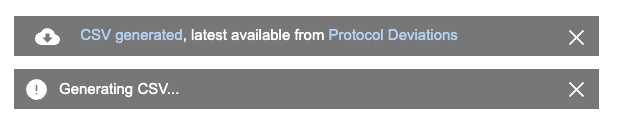
- From the Protocol Deviation menu (), select Generate CSV.
- Workbench generates the CSV. When finished, Workbench displays a notification with a link to download the file.
Once you or another user has generated a CSV for the listing, you can also download it from the Protocol Deviations page, without opening the listing.
To download it there, click Download (). You can hover over the icon to see who generated the CSV and when.