About the Clinical Operations - EDC Connection
With the Clinical Operations - EDC Connection, you can easily leverage operational data from your EDC vault for operational reporting within your CTMS (Clinical Operations) vault. CTMS users can then report on subject statuses and status dates captured in Veeva EDC, as well as navigate from a Subject within Veeva CTMS to a subject’s Casebook in Veeva EDC. Once configured, you can share data between Veeva EDC and CTMS automatically, instead of manually exporting and importing data.
You can define subject milestone exports in Veeva EDC that update subject information in your CTMS Vault. This export contains the following: Study, Country, Site, Subject ID, Subject Status, Screened Date, Screen Failed Date, Randomized Date, Enrolled Date, Withdrawn Date, End of Treatment Date, End of Study Date. With this feature, you can easily keep concurrent data for your subjects in both your EDC and CTMS vaults.
If you have a Clinical Operations - EDC Connection , any closeout PDFs you generate are automatically sent to eTMF if the following conditions are met:
- The Clinical Operations - EDC connection is active.
- The study is connected to Clinical Operations.
- The CDMS to CTMS Site Closeout PDF Data integration is active (this integration is active by default).
- Your organization is using eTMF.
If these conditions are met, every time you generate Closeout PDFs for a site, a new version of the PDFs is generated and sent to eTMF. This process is automatic, and there are no additional steps to be performed on your end.
This feature is only available to organizations with both CTMS and EDC vaults.
Connect via FTP or Spark
Depending on your vault’s configuration, you can connect your CTMS vault to your EDC vault using FTP and the Clinical Loader, or via a Spark connection. To integrate using a Spark connection, the EDC to CTMS Spark Integration feature must be enabled in your EDC vault.
While the FTP connection method continues to function, the 20R1 (April 2020) introduced a new, standard EDC/CTMS connection using Spark Messaging. This is the recommended path to transfer record data.
How the Connection Works via Spark Connection
When you use a spark connection to integrate with Vault CTMS, Veeva EDC and Vault CTMS can automatically exchange record data in near-real time.
For example, whenever a data entry user creates a new Subject in EDC (by creating a new Casebook in the Data Entry tab), CTMS automatically creates a new, matching Subject in the CTMS vault.
The EDC/CTMS Spark connection exchanges the following data:
From Vault CTMS to Veeva EDC:
- Studies (updates only, Studies must still be manually created and linked in Veeva EDC)
- Study Countries
- Study Sites
From Veeva EDC to Vault CTMS:
- Event Definitions (into the Visit Definitions object in Veeva CTMS)
- Subjects
- Events (into the Subject Visits object in Veeva CTMS)
- Protocol Deviations (CTMS doesn’t return any Protocol Deviation data to EDC)
- Procedures
- SDV
- DMR
For SDV and DMR, the connection directly retrieves Event review status roll-up calculations from Event Operational Summary records in EDC, including data for log events.
Setting Study Object Fields
The EDC/CTMS Spark connection enables Vault to create and update records in a connected vault after a user makes a change to an affected record in the source vault. Once an Admin activates the connection between EDC and CTMS, record data begins flowing between the vaults based on the Connect to Vault CDMS and Connect to Clinical Operations fields on the Study object:
- For Studies in CTMS that have this field set to Yes, Vault creates or updates Studies*, Study Countries, and Sites in EDC as you create or update those records in CTMS.
- For Studies in EDC that have the Connect to Vault Clinical Operations field set to Yes, Vault creates or updates Subjects, Subject Visits (Events), and Visit Definitions (Event Definitions) in CTMS as you create or update those records in EDC.
The connection only updates Studies. It cannot create Study records.
Veeva EDC has site uniqueness requirements that differ from CTMS. Creating records through the Connection can bypass an object’s uniqueness configuration in EDC. When a record is created manually through the UI, the object’s uniqueness still applies.
See Configuration for the EDC/CTMS Spark Connection for configuration instructions.
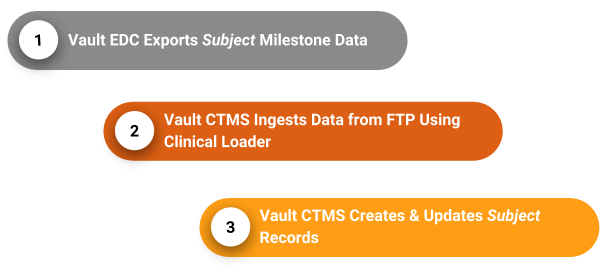
How the Connection Works via FTP
With this connection, Veeva EDC exports subject data in a CSV-formatted View Set to an FTP server as part of a scheduled, recurring job. Vault CTMS then ingests that data from the FTP server with Clinical Vault Loader into the CTMS vault, and creates new Subject records or updates existing Subject records accordingly.
See Configuration for the EDC/CTMS FTP Connection for configuration instructions.
Entering data in the CTMS vault does not update related data in the EDC vault.
Subject (CTMS) Object Fields
Data from Veeva EDC populates the following fields on the Vault CTMS Subject (subject__clin) object:
- Subject ID
- Subject Status
- Screened Date
- Screen Failed Date
- Enrolled Date
- Randomized Date
- Withdrawn Date
- End of Treatment Date
- End of Study Date
- EDC ID
Actions within Veeva CTMS
When configured, users with access to both EDC and CTMS vaults can open a Subject’s casebook in EDC directly from their CTMS vaults. What actions those CTMS users can perform in EDC depends on their permissions. For example, a CRA could navigate to a casebook and perform source data verification (SDV) in their EDC vault, while a data manager might open a casebook to create a query.