New Features in 23R1.3
Procedures Enhancements, Send SDE to CDB, and more...
Release Date: June 9, 2023
We are pleased to bring you the following new features in this week's release. See a summary of feature enablement for this release below. Information on developer features (REST API) is in the Developer Portal.
CDMS
Features in this section are changes that apply to all application areas of Vault CDMS.
Show Sponsor in the My Vaults Page
Use Case
Veeva CDMS Customers can have access to multiple Vault applications and CRO end users often manage and operate across multiple sets of separate Sponsor Vaults including (Dev, Test, Prod and Train). The new My Vaults page provides a better user interface through an enhanced grid view and quick navigation for all Vault users with access to many Vaults.
Description
This feature provides an enterprise-wide, homepage grid view that allows scalability for users who need to navigate and manage tasks across higher numbers of Vaults (500+). Columns within the home page can be sorted, filtered, and searched.
Users can select box or grid view which will display the following columns:
- Vault Name
- Sponsor
- Last Login
- Application Family (e.g. Clinical, Promomats, RIM, Quality, etc.)
- Type (e.g. Sandbox, Production, etc.)
- Domain
- My Tasks
Column names are translated based on user’s language preference.
Enablement & Configuration
This feature is automatically available for domains where the MyVaults flag is set by Veeva Admins. The Sponsor and Type objects are only editable by Vault Owners. Filters display by default for users with more than 25 Vault memberships.
Small Screen Size Warning
Description
Vault presents users with a warning dialog in cases where a user’s screen resolution is less than the minimum supported setting of 1280 x 720. The dialog displays to impacted users upon entering the Data Entry, Review, Protocol Deviations, and Assessments tabs for the first time in a session. As part of this change, we made small changes to the shades of some colors in the application.
Enablement & Configuration
These changes apply automatically.
Data Entry
Features in this section are changes to the Data Entry tab, a working area for investigators and clinical research coordinators to enter study execution data.
All Bookmarks in Detail PDFs are Clickable
Description
In the Detail PDF, all bookmarks are now hyperlinks.
Enablement & Configuration
This feature is automatically enabled.
Form Not Submitted Dialog to Display Only for Users with Data Entry Permissions
Use Case
The Form Not Submitted Dialog will only display to affected users.
Description
The Form Not Submitted dialog will only display to users with Data Entry permissions when they navigate away from an In Progress form.
Enablement & Configuration
This feature is automatically enabled.
Clinical Coding
The following are new features for Coder, the clinical coding area for Vault Coder.
Coder: Display Verbatims and Related Items in ALL CAPS
Use Case
With this feature, Coders can quickly and easily read Verbatims when they are grouped regardless of capitalization. Previously, coders would see duplicates of the same Verbatims and Related Items due to differences in capitalization.
Description
With this release, Verbatims are displayed and grouped in all caps in List View, Group View, and the Properties Panel when the respective study setting is set to “Yes.” The Coder Verbatims page will display the following fields in all caps format only:
- Verbatim
- Seriousness (if applicable)
- Indication (if applicable)
- Route (if applicable)
- Any other configured related items
When grouping by these fields, Coder will group in all caps format only.
Enablement & Configuration
This feature is automatically enabled.
Study Design & Configuration
Features in this area apply to Studio, the study design and configuration area for Vault EDC.
Create Procedures via Rules
Use Case
This feature supports a more granular definition of Procedure completeness when automating procedure records to CTMS.
Description
Procedure definitions can be configured by Study Designers in the new Integration Configuration tab within Studio. The creation of the procedure records can be based on field mappings or by creating user defined rules to evaluate casebook data. Procedure definitions can evaluate eCRF data values, subject status, form submitted date or form status, such as SDV, and also per the subject’s country. Procedures can be determined from a single or repeating form design or from a repeating event group. Configurations can now be validated in TEST as part of user acceptance testing without transmitting test data to CTMS. We added a new Integrations sheet to the SDS and Difference report for studies using this feature.
Enablement & Configuration
This feature is automatically enabled for new studies. For existing studies please contact Veeva Managed Services to enable the appropriate setting.
Learn More
Rules Enhancements
Use Case
These rule enhancements reduce errors and provide usability improvements, further validations, and additional rule configurations for Study Designers.
Description
The following enhancements have been made to rules:
- Subject status rules now allow users to use repeating Event Groups, Forms, and Item Groups in the Rule Action, so long as a sequence number is specified.
- Added functions:
HasDuplicates(): Returns true if any 2+ arrays have equal values for all the identifiers insideSiteDateTime(): Automatically creates datetime from Date item and Time item in Site timezoneSiteDateValue(): Returns the date from a datetime item and automatically puts it in Site timezone
- The Rule Preview job now shows all rules from the job in the rule_details file, to provide clarity for job results where a rule was included in the job but resulted in no change (e.g. Action = None).
- General enhancements:
- Study Designers will see updated rule action labels and order of actions when selecting a Rule Action in the rule editor.
- Query action has been relabeled to Open Query
- Ready to Randomize has been relabeled to Set as Ready to Randomize
- A new rule action type called Create Integration Entry has been added to support new Procedures features
- When changing the Evaluate Rule When property for a rule, the rule editor no longer clears Rule Actions if a valid action has already been set
- In the SDS, the Date Comparisons tab has been relabeled to Comparison Rules to match Studio
- Study Designers will see updated rule action labels and order of actions when selecting a Rule Action in the rule editor.
Action Required: Users must fix or inactivate misconfigured rules (specified below) in order to deploy from DEV. Studies that have already been deployed to UAT/PROD won’t be affected but misconfigured rules will be flagged as invalid in the next casebook version.
Additional validations have been added to safeguard against misconfigured rules, including:
- Derivation rules where the result does not match the target item’s data type
- Rules using @Form or ‘this form’ identifiers when the associated form in the Rule Details panel doesn’t match in the expression or action
- Subject status rule actions must be fully qualified
We also downgraded ER-46, ER-20, and ER-19 to warnings. This change is not part of 23R2 pre-release and will only apply at the 23R2.0 general release.
With this release, several functions from the Vault Platform are now exposed in the Rule Editor’s Formula Selector. Many of these functions aren’t applicable to the formulas most commonly used for CDMS rules. We recommend that you only use those functions covered in the CDMS Formula Reference.
Enablement & Configuration
Automatically enabled for new and existing studies. End users must revise or inactivate any broken rules the next time they validate study configurations.
Learn More
Study Administration
Features in this section apply to EDC Tools, a study-level administration area for Vault EDC.
Job Governor Enhancements
Use Case
This feature ensures that multiple jobs for the same type of data aren’t scheduled to run concurrently.
Description
The Audit Trail Export jobs (by Study, by Site, and by Subject) will now be included in the Job Governor. This inclusion ensures that two audit trail jobs of the same type won’t run simultaneously, freeing resources for other jobs. For audit trail jobs, this feature will only keep ad hoc jobs from starting if a job of the same type is running.
Users cannot start any identical extract job covered by the job governor if the job is in the Queued or In Progress status with this feature.
As part of this feature, we introduced the Queued status in the extract job governor to improve processing efficiency.
Enablement & Configuration
This feature is automatically enabled.
Learn More
Deploy from Template Vault
Use Case
Previously, any changes made to the CRO standard vault settings needed to be updated separately in each of the CRO-managed Sponsor vaults. With this feature, users can push changes from the CRO Template vault into multiple Sponsor DEV vaults at the same time.
Description
In System Tools, CRO users with the Manage Deployments permission can now create and edit Deployment Groups in order to deploy role and permission updates from the CRO Template Vault into multiple Sponsor-DEV vaults at the same time. Users can include up to 15 vaults in a single Deployment Group.
Enablement & Configuration
Contact Veeva Support to enable this feature in your vault.
Learn More
Event Window Reporting Enhancements
Use Case
This update addresses inconsistencies in calculation caused by daylight savings time. It also completes the transition away from datetime fields for Event Date and Planned date in support of the planned deprecation of event datetime fields.
Description
This release updates the calculation for Days Outside Event Window and Event Window Status in the Event Progress Listing and Event Operational Summary report. The calculation now uses Event Date and Planned Date, rather than Event Datetime and Planned Datetime
Additionally, new Event Window Statuses have been added with this release, which will be visible in the Event Progress Listing and event reports.
- Planned: An event does not yet have an event date.
- No Defined Window: An event window has not been defined for the event.
Enablement & Configuration
This feature is automatically enabled.
SDE: Enhancements
Use Case
These updates provide additional control over the Study Data Extract output, as well as additional metadata.
Description
The following enhancements have been made to the 23R2 version of the SDE:
- New option to configure which item display label (default label or short label) will be included in the extract. If no short label exists for an object, the default label will be used. This label can be found in the study definitions file and is used as the SAS label. The allowed length of the short label was also increased to 40 characters to support this update.
- The Export File Type field is now a selector that allows any combination of the three options: CSV, SAS, and XPT
- Added Item External ID column to the SYS_ILB, SYS_Q, SYS_PD, and SYS_LINKS datasets
- Added Event Group External ID column to clinical datasets, SYS_EVT, SYS_FORM, SYS_ILB, SYS_LINKS, SYS_Q, and SYS_PD
- Added columns to the SYS_LINKS dataset to clarify the Form Created Date and Link Created Date
- Users must have the View Casebook permission to see and run the SDE job
Enablement & Configuration
This feature is automatically enabled with SDE version 23R2.
Clinical DataBase (CDB)
The following are new features for the CDB application, the Vault CDMS solution for data cleaning and reporting.
Availability: Clinical DataBase (CDB) is only available to CDB license holders. Contact your Veeva Services representative for details.
Advanced Joins in CQL
Use Case
The ability to define the direction of table joins allows for more complex and performant listings, where users can define with more precision which rows of data to return.
Description
CQL now supports the definition of direction and criteria for table joins. CQL allows multiple joins within a single CQL statement.
In this release, CQL supports the following join types:
LEFT JOIN: All records from the first (left) table and any matching data from the second (right)RIGHT JOIN: All records from the second (right) table and any matching data from the first (left)INNER JOIN: Only records from both tables where a match is found
Users must define the join conditions for advanced joins, including joins to the header. Tables of data can be a form, subquery, or a view.
Enablement & Configuration
Advanced joins are automatically available.
Learn More
CDB Product Naming
Use Case
This change improves the understanding of the different components of the application through consistent naming.
Description
With this release, we’ve updated the header of CDB Workbench and CDB Manifest Builder with logos that reflect their names. As part of this change, we updated the URL of CDB Manifest Builder. The previous URLs will continue to work after the release, redirecting the user to the new URL.
Enablement & Configuration
These changes apply automatically.
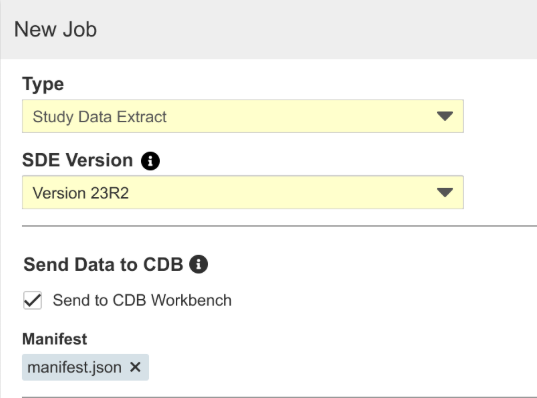
SDE: Send Data to CDB
Use Case
This feature allows Vault data that isn’t included as part of form data currently transferred via the Workbench export to be sent on a regular schedule to CDB.
Description
With the 23R2 version of SDE, customers with CDB enabled can send SDE datasets to CDB, where they’ll be handled like third-party data in the application. Users can schedule jobs or run them ad-hoc, as well as include specific datasets in the job. SYS datasets with metadata not generally available from EDC and custom object data can be included when configured in the Vault, for example. Note that sending SDE data to CDB allows two additional SDE jobs to be scheduled and doesn’t count against the job governor limit. Attaching a manifest file to the job is required, as the data will be treated like third-party source data in CDB.
Enablement & Configuration
Contact Veeva Support to enable this feature.
Learn More
Integrations
Features in this section are new integrations with Vault CDMS or enhancements to existing integrations.
Safety Integration: Study Drug Level Blinding
Use Case
Studies may involve more than one study drug, with different blinding requirements. This feature allows organizations to handle those differing requirements when sending study drug information to the safety system.
Description
Safety Integrations (formerly E2BLink) now classifies the blinding property at the study drug (treatment) level. Prior to this release, blinding was a study level settings. This could cause issues when a study has multiple study drugs, where one is blinded and one is not. This blinding is controlled by the Blinded field in the Study Drug section of Tools > EDC Tools > Safety Configuration.
As part of this feature, we removed the Investigational Product Blinded setting from the General Settings section, as this has been replaced by the new Blinded property in the Study Drug section.
Enablement & Configuration
This feature is automatically enabled. Existing studies with a configured study drug will automatically have the blinding property set to No (false).
Safety Integration: Handle Adverse Events with no Term Item Value
Use Case
Prior to this release, import into some safety systems fails when the Item for the term or verbatim is empty on the Adverse Event form.
Description
Safety Integrations (formerly E2BLink) will now add a default AE term or verbatim where the event in the case no longer has text. This text is “AE term no longer available”. The primary example of this is a form reset by site on mistaken entry of AE form.
Enablement & Configuration
This feature is automatically enabled.
Safety Integration: Enhanced Reporting
Use Case
A single report is crucial for the review of safety cases in a study. Previously, one had to cross reference a number of reports and view casebooks in the Data Entry tab to align which Safety Cases and Safety Messages applied to which subject and serious adverse events. Additionally, some safety systems have quick searches by file name sent to them, and showing that information at the safety case level will be useful.
Description
Safety Integrations (formerly E2BLink) now include additional fields on the Safety Case for better tracking and referencing. These new fields are only tracking information from the release forward, older safety cases are not updated with information until there is another follow-up generated for the case.
We added the following columns to all existing and new EDC reports on Safety Cases and Safety Messages:
- Events in Case: All transferred cases.
- Form Sequence: The Sequence Number of the SAE form.
- Last Send File Name: Filename of the last sent file. Users can search the safety system for the file.
- Last Send Safety: Date and time of the most recent Safety Message.
- Last ACK File Name: The file name with the date and time.
- Additional Follow-up Pending: This column shows “true” if additional changes were detected, but a previously sent follow-up hasn’t yet triaged into the system.
This new tracking information is only available for Safety Cases and Safety Messages sent from 23R2 and forward.
Enablement & Configuration
This feature is automatically enabled. The template reports in the system for Safety Cases and Safety Messages now contain the new fields. To use these fields in any custom reports, a user will need to add them.
Learn More
Safety Integration: Vault Safety Inbox
Use Case
Connecting safety data from Vault CDMS studies to Vault Safety requires one time configuration in both Vaults. In addition to the one time connection setup (Vault to Vault), a study in Vault CDMS can be optionally pointed to either of the Vault Safety Inbox locations.
Description
Safety Integrations (formerly E2BLink) includes new additional support with the Vault Safety Inbox, for those integration studies with Vault Safety. Both the Vault Safety and Vault CDMS systems still need one time setup for the connection, as before. But, the ability to direct safety data to the Inbox (vs. AER Inbox) tab no longer needs to be configured on the Vault Safety side. Instead, it is a setting within the CDMS Vault that directs the data to the newer Inbox tab.
Enablement & Configuration
This feature is enabled automatically. By default all vaults are set to use the AER Inbox for Vault Inbox Type. This is the default behavior from prior to this release. For organizations using the main Safety Inbox, connection properties in CDMS must be adjusted.
Safety Integration: Nullification Mapping & Default Reason
Use Case
Nullification follow-up transfers are when a case / event(s) are no longer applicable. Previous to this release, Vault CDMS was not sending anything for reason, and some safety system configurations consider it required, Examples of nullification transfer:
- Site data entry mistakes, for example, a form reset, where a case previously sent,
- Downgrading an existing serious AE to non-serious, where a case previously sent.
Description
Safety Integrations (formerly E2BLink) now allows mapping to a Reason for Nullification / Amendment (C.1.11.2) from the Serious Adverse Event form. For studies that don’t have this mapping, and a case is nullified, Vault CDMS will add a default reason to the E2B XML sent to the safety system.
Enablement & Configuration
This feature is available automatically, but a study designer must update the study design with a mappable Item in a non-repeating Item Group and deploy that change.
Screen Subjects Outside CDMS
Use Case
For certain studies, using IXRS and CTMS, the desired workflow is to first process a subject’s pre-screening and screening events in CTMS and then only add the subject in CDMS once they are enrolled into the study. This feature removes the mismatching that can occur between CDMS and CTMS by creating only enrolled subjects in CDMS and automatically connecting them with CTMS.
Description
EDC APIs now have the option to exclude subjects that have not reached an enrolled status in CTMS. Prior to enrollment, subjects will first be created in CTMS, either manually or through an integration with an external system. Once enrolled, the subject will be created in CDMS by the IXRS system and the connection with CTMS will check and match the corresponding subject record in CTMS. The subject’s status will reflect as “Enrolled” in CDMS per the Subject Status rules configured in Studio by the Study Designer.
Enablement & Configuration
This feature is automatically enabled for studies that are using the 23.2 version of the APIs and where CTMS or a third party system are connected. Existing Live studies would need to update their API version to utilize this feature. To see the new IXRSID field in the SYS_SUB dataset, the 23R2 version of the SDE must be selected when running the extracts.
EDC Migrator
Features in this section are new features for Veeva EDC Migrator.
Feature Enablement Summary
| Feature Name | Configuration | Dependencies | Day 1 Impact to Primary Users | Users with Day 1 Visibility |
|---|---|---|---|---|
| CDMS | ||||
| Show Sponsor in the My Vaults Page |
|
|||
| Small Screen Size Warning |
|
|
||
| Data Entry | ||||
| All Bookmarks in Detail PDFs are Clickable |
|
|||
| Form Not Submitted Dialog to Display Only for Users with Data Entry Permissions |
|
|||
| Clinical Coding | ||||
| Coder: Display Verbatims and Related Items in ALL CAPS |
|
|
||
| Study Administration | ||||
| Job Governor Enhancements |
|
|||
| Deploy from Template Vault |
|
|||
| Event Window Reporting Enhancements |
|
|||
| Study Design & Configuration | ||||
| Create Procedures via Rules |
|
|||
| Rules Enhancements |
|
|||
| Vault CDB | ||||
| Advanced Joins in CQL |
|
|||
| CDB Product Naming |
|
|||
| SDE: Send Data to CDB | Support | |||
| Integrations | ||||
| Safety Integration: Study Drug Level Blinding |
|
|||
| Safety Integration: Handle Adverse Events with no Term Item Value |
|
|||
| Safety Integration: Enhanced Reporting |
|
|||
| Safety Integration: Vault Safety Inbox |
|
|||
| Safety Integration: Nullification Mapping & Default Reason |
|
|||
| Screen Subjects Outside CDMS |
|
|||
| EDC Migrator | ||||
| Limited Availability: In the current release, EDC Migrator is only available to early adopter customers. Contact your Veeva Services representative for details. | ||||
Enablement Legend
- Configuration: This field lists the location(s) where configuration for this feature occurs, for example, "Studio" or "EDC Tools". "Support" indicates that this feature must be enabled by Veeva Support, and "Vault Admin" indicates that configuration must be performed by a Vault Owner in the vault's Admin area.
- Dependencies: This field lists any dependencies required to use this feature, for example, Labs or Expression Engine V2. The other columns assume that the dependencies are enabled/in use.
- Day 1 Impact to Primary Users: This feature is visible and available to one or more primary user teams (Site Users, Clinical Team, and Coders) on day 1. Otherwise, this feature is either only visible to study designers or administrator users, it requires configuration before it is visible to primary users.
- Users with Day 1 Visibility: This feature is visible to these users on day 1 if no configuration occurs.