New Features in 19R2.3
Track Queries Leading to Data Changes, Veeva Learning Integration, and more...
Release Date: October 11, 2019
We are pleased to bring you the following new features in this week's release. See a summary of feature enablement for this release below. Information on developer features (REST API) is in the Developer Portal.
CDMS
Features in this section are changes that apply to all application areas of Vault CDMS.
Audit Trail Enhancements
Use Case
Users can have a smoother experience viewing audit trail entries.
Description
With this release, we made the following visual enhancements to the audit trail:
- We updated action labels for accessing the audit trail so that they are consistent across objects and across all of CDMS. These actions are now labeled with the level of audit (Casebook, Event, Form, etc.) and “Audit Trail”. For example, users can select Casebook Audit Trail to view the casebook-level audit trail.
- We increased the size of the Audit Trail dialog so that it can display more information without text wrapping.
Enablement & Configuration
These changes apply automatically with no additional action required.
Detail PDF Enhancements
Use Case
The new PDF redesign addresses some of the navigational submission requirements and also improves readability for end users.
Description
In this release, we redesigned the Detail PDF to provide better navigation and readability for end users.
Each page in the Subject Data PDF will now include a table header that provides details about the form being displayed, such as Event, Event Date, Form, and Form Version. The Detail PDF only includes the necessary information required for submission (SDV, DMR, Freeze, and Lock have been removed).
With this release, we also added PDF bookmark sections to allow the user to navigate to By Visits or By Form to improve the user’s navigational experience. In the By Visit view, the user will see all of the subject events, which are ordered by study design and contain all forms corresponding to that event. In the By Form view, all unique forms are ordered alphabetically.
If the PDF includes Audit Trail, there will be two additional sections called Audit Trail by Visit and Audit Trail by Form, which will be formatted in the same way as the By Visit and By Form sections. If the PDF includes queries, there will be a bookmark section called By Queries.
Enablement & Configuration
This feature is available to existing Studies by configuration. Contact Veeva Services for details. For all Studies created after this release, this feature is automatically enabled, with no additional configuration required.
Data Review
Features in this section are changes to the Review tab, a working area for clinical research associates and data managers, or to review functionality within the Data Entry tab.
Review UI Enhancements
Description
In order to make the site filter consistent in both the Study Sites and Queries sections in the Review tab, the filter label at the top of the listing grid now displays Site Number in both sections. The Site Number header label has also been updated from “Site #” to “Site Number.” Additionally, Users can hover over the help icon to view help text in the Review tab.
Enablement & Configuration
These changes are automatically applied in all vaults where the Review tab is enabled. If the Review tab is not enabled in your vault, and you would like to enable it, contact Veeva Support.
Removed Review Status Propagation
Use Case
This change provides better system performance and increases scalability.
Description
To improve long-term system performance, Vault will no longer propagate review status (SDV, DMR, freezing, locking, and signature) throughout the execution data model for Studies that start after the 19R3 release. In this new model, the review status for each review is tracked at the Item level, and Vault displays the correct status at the Form and Event level based on those Items. Vault uses Review State records to track the review statuses of each Item, instead of status fields on the Item object record. This change also provides the appropriate infrastructure for potential future enhancements.
As part of this enhancement, we added the following standard report templates:
- Standard Template: Form SDV Summary (v2)
- Standard Template: Form SDV Detail (v2)
- Standard Template: Form SDV Monitoring Activity per Week (v2)
- Standard Template: Form DMR Summary (v2)
- Standard Template: Form DMR Detail (v2)
- Standard Template: Form DMR Monitoring Activity per Week (v2)
Enablement & Configuration
This feature is not available for existing Studies. It is only available for Studies created after this release. The new standard report templates are available automatically.
Vault Coder
The following are new features for the Vault Coder application, the Vault CDMS solution for clinical coding.
Ability to Change the Dictionary for a Form
Use Case
With this enhancement, customers now have options to address issues they may encounter when configuring their study for use with Vault Coder.
Description
With this release, Vault now locks the Coding Configuration for a Form once Code Requests exist for that Form. Coding administrators can upversion a Form to a new dictionary version at any time from Coder Tools. However, if an organization needs to switch to an earlier version of a dictionary or change the dictionary type (from WHODrug to MedDRA or vice versa), they can now replace the coding configuration for a Form in Studio. An organization can contact Veeva Support to remove existing Code Requests and delete the form’s coding configuration. Then, a study designer can reconfigure the Form with the new Dictionary Release selection.
When the updated configuration is complete, a lead data manager can run the Autocoding and Suggestions job to create Code Requests for all existing, completed Forms in EDC. If there are matching records in the assigned Synonym List, Vault will also autocode those newly created Code Requests.
Note that unless this feature is utilized, if the target Study has a different Dictionary Release for a form than the source Study, a deployment will fail.
Enablement & Configuration
This feature is available automatically in all vaults containing the Coder application, with no additional configuration required.
Coder General Configuration Enhancements
Use Case
Coders can navigate Coder and assign codes more quickly.
Description
Vault Coder no longer displays the study’s Therapeutic Area. We removed it because there is no way of setting this field value outside of Admin > Business Admin. This change increases customer’s configuration independence.
Enablement & Configuration
This feature is available automatically in all vaults that include the Coder application.
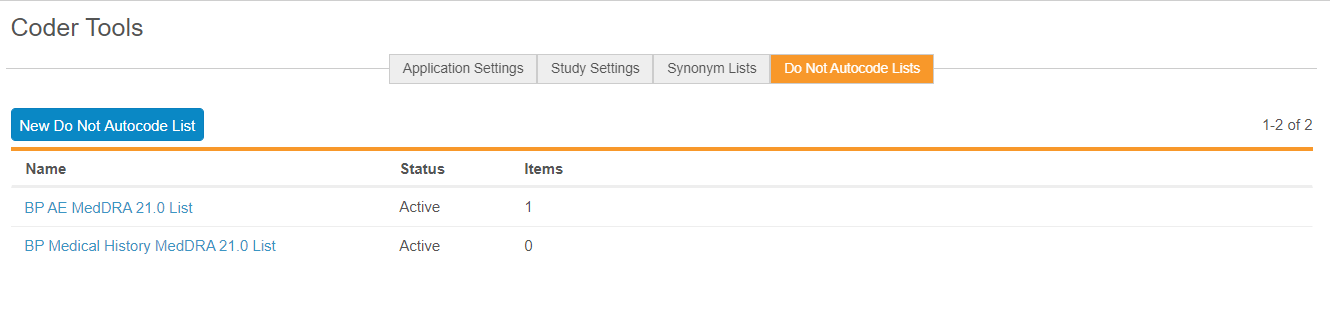
Renamed "Stop Lists" as "Do Not Autocode Lists"
Use Case
This change makes the Do Not Autocode List feature easier to understand.
Description
With this release, we renamed Stop Lists as Do Not Autocode Lists to better communicate what this object does. When a verbatim is on a Do Not Autocode List, Vault does not attempt to autocode any Code Requests with that verbatim.
Enablement & Configuration
This feature is available automatically in all vaults containing the Coder application, with no additional configuration required.
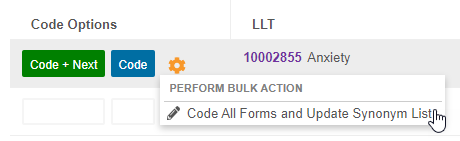
Extend a Coding Decision to All Forms & Update the Synonym List
Use Case
When there is a new dictionary release or when a coding group adopts a new philosophy, the coding group can use this feature to easily standardize the new coding decision across all matching Forms.
Description
From the Code Request Listing page, a coder can choose to update a coding decision on all Forms that are assigned to the same Synonym List with the new Code All Forms and Update Synonym List action. They can also choose to have any matching records in the Synonym List updated to match their new coding decision.
In support of this feature, we added an Actions menu to each row in Suggestions and Dictionary. Hover over a dictionary term or suggestion to display the Actions menu.
Enablement & Configuration
This feature is available automatically in all vaults containing the Coder application, with no additional configuration required.
Dictionary Search Enhancements
Use Case
Coders can retrieve more relevant results quickly and without the need to rephrase their search term.
Description
With this release, we enhanced the ability of Dictionary Search to retrieve relevant results when users search for terms in different orders or for terms that are missing leading characters.
Enablement & Configuration
This feature is available automatically in all vaults that include the Coder application.
Reports & Dashboards
The following are new features for reports and dashboards in Vault CDMS.
Track Queries Leading to Data Changes
Use Case
The ability to report on query efficiency enables study designers to more easily understand if their rules are effective and if they should consider creating the same check in their next Study.
Description
Vault now tracks whether or not a system-generated query caused a user to change an Item value with the new Query Summary object. This calculation checks if the Item value’s modified date and time is between the times that the query was opened and closed.
Organizations can use the new Queries Causing Data Value Change report to view data or create customized reports showing this metric to study designers and other interested users. In this initial implementation, there are some limitations:
- Vault can only track changes to the Item containing the query. For example, if a rule creates a query on Item 1 when Item 1 is “Yes” and Item 2 is “No”, and a user edits Item 2, Vault does not track the data change. If the user had edited Item 1, Vault would have recorded the data change.
- Vault does not track whether or not manual queries resulted in data changes.
Enablement & Configuration
This feature is available automatically in all vaults, with no additional configuration required. If an organization wants to customize a report on query efficiency, they can copy and edit the Queries Causing Data Value Change report.
Enhanced Coder Metrics
Use Case
Customers can use these new metrics to track coding team performance.
Description
We added the Last Coded By field to the Medical Coding Request object and the Last Queried By field to the Medical Coding Request Query object to allow users to create reports and dashboards related to coding progress and query status in Coder.
Enablement & Configuration
This feature is available automatically in all vaults containing the Coder application. Customers can update their existing reports or create new reports to show data from these two new object fields.
Assessments
The following are new features for the Assessments area of Vault EDC.
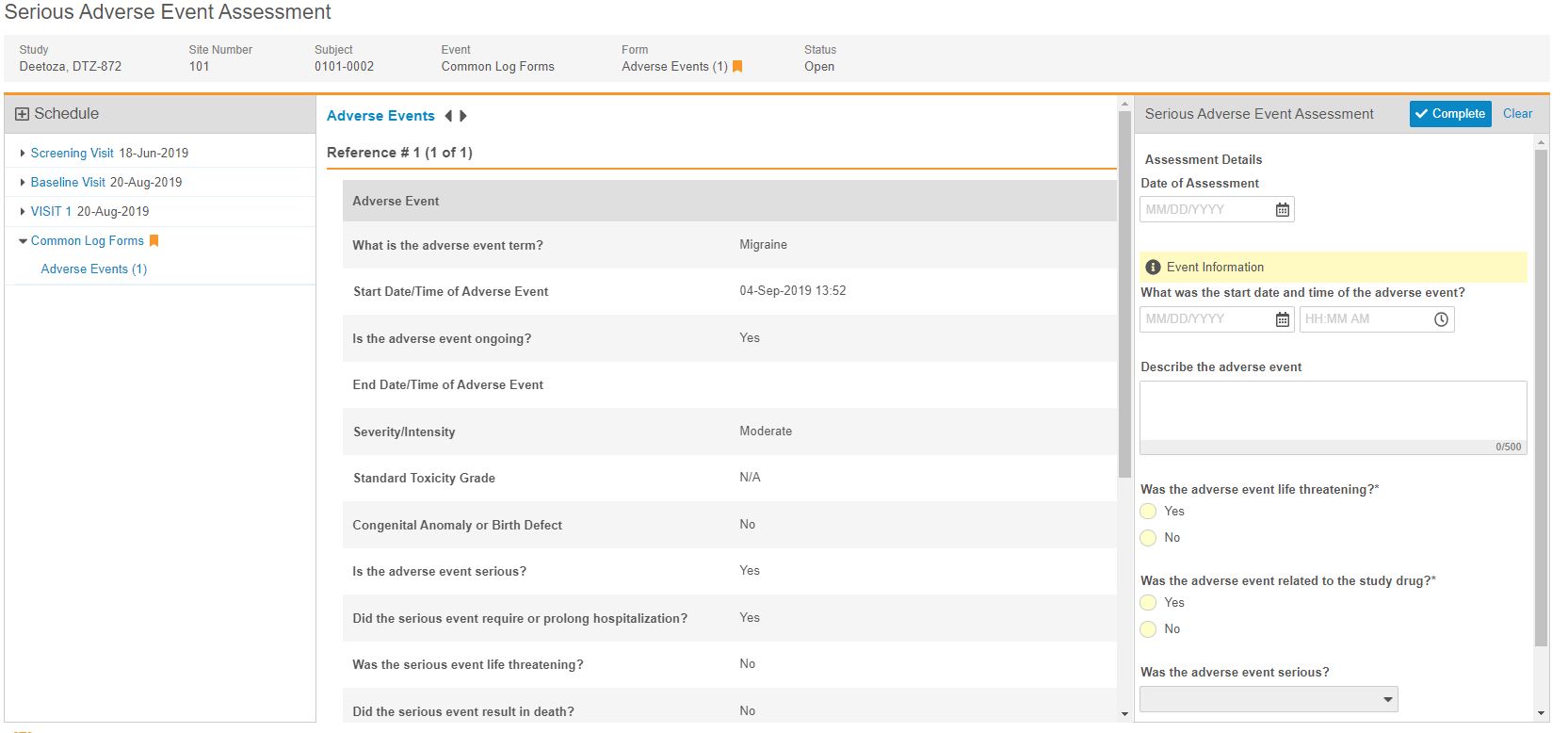
Clinical Review Assessments
Use Case
Clinical review assessments allow organizations to collect data outside of a Casebook while still maintaining the data’s relationship with the Subject.
Description
During a study, there may be data collection requirements that are not part of a casebook, but still related to a subject. With this release, Vault CDMS now supports this requirement with clinical review assessments. Clinical review assessments collect data and display a limited set of read-only data from a subject’s Casebook, called “supplemental data”. After an Assessment is completed, Vault stores a snapshot of the supplemental data, so that anyone who reviews the assessment can see the data that was used during the assessment, even if that data has since changed. Clinical review assessments do not support queries, signatures, SDV, or DMR. Users with the appropriate permissions can download both the assessment and the supplemental data as a PDF file for offline viewing.
Studio users can design multiple types of Assessments for a Study, easily adding questions to the assessment and choosing which Forms and Items should be available as supplemental data. Then, a lead data manager can assign those different Assessments to different Study Roles from EDC Tools > Assessments.
This feature adds the new Assessments tab, and Assessments subtabs to Studio and EDC Tools. This feature also adds the standard CDMS Clinical Assessment Viewer and CDMS Clinical Assessment Editor study roles and the following new permissions:
- Assessments Tab
- View Clinical Assessments
- Edit Clinical Assessments
- Manage Assessments
Enablement & Configuration
Contact Veeva Support to enable this feature in your vault.
Study Design & Configuration
Features in this area apply to Studio, the study design and configuration area for Vault EDC.
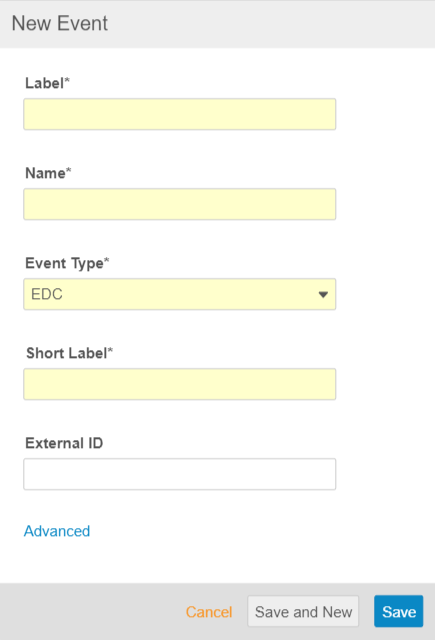
Studio Usability Enhancements
Use Case
These enhancements reduce study build time by simplifying common tasks.
Description
With this release, we made the following usability enhancements to Studio:
- We refreshed the New Event dialog to allow users to enter more information in the dialog, instead of providing that information later in the Properties panel. Users can click Save and New to save their new Event and then immediately begin creating another one.
- When copying Forms, a user can now select all Forms within a source study by selecting the header checkbox. If the user filters the list of available Forms, selecting that checkbox selects only those filtered Forms.
- We made several small usability improvements, such as no longer defaulting the Repeat Maximum property to “9999”. Instead, this field is now blank by default.
Enablement & Configuration
This feature is available automatically without any additional configuration.
New Studio Navigation Pattern
Use Case
These changes allow users familiar with other vault applications to have a similar experience, which reduces the amount of training required to use Studio.
Description
With this release, we overhauled the look and feel of Studio for two main purposes:
- To provide Studio with a more Vault-like navigation pattern between the types of objects.
- To allow for navigation between Studies on the existing, manual deployment model (import and export) and the new, automated deployment model.
These changes include:
- Studio now opens to a list of Studies, instead of a list of Organizations.
- We replaced the tabbed navigation interface with a vertical navigation panel on the left side.
- We removed the Browse and Design views and buttons. Instead, users can click Schedule in the left navigation panel to open their schedule, and then use a Back to Study button to return to Studio.
- The Properties panel now only displays when in use, sliding out from the right-hand side, instead of permanently. This allows users to view more columns in the list without horizontal scrolling.
- We added additional columns to the object record list views.
- We updated the breadcrumb menu to allow users to easily navigate between their Study Masters and their legacy Studies.
Enablement & Configuration
These changes apply automatically with no additional configuration required.
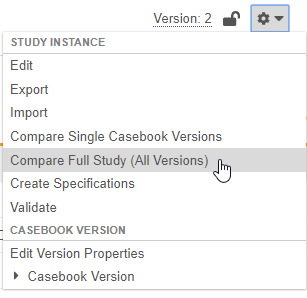
Compare All Versions in Studio
Use Case
This enhancement reduces the need for manual creation of difference reports and provides more clarity when deploying studies or when attempting to build validation reports study changes.
Description
With this release, Studio users can now compare all versions of two separate Studies in a single report. A user can choose to compare two studies with the new Compare Full Study (All Versions) action in the Studio Actions menu.
For this report, Vault generates a ZIP file containing a series of Excel™ workbooks, one for each version included in the comparison, and then an additional file containing information about “versionless” objects, such as study settings and Review Plans.
As part of this enhancement, we relabeled the action to compare single versions as Compare Single Casebook Versions in both Studio and EDC Tools.
Enablement & Configuration
This feature is available automatically in Studio, with no additional configuration required.
Compare Forms Across Studies
Use Case
This enhancement reduces the need for manual creation of difference reports and provides more clarity when deploying studies or when attempting to build validation reports based on changes between a version and the library.
Description
Studio users can now perform library comparisons by selecting the new Exclude Event Group and Event Differences when comparing casebook versions. This allow a Form-level and below comparison between two versions, ignoring their location on the study schedule. This comparison report still includes the Schedule worksheet but ignores any differences between Event Groups and Events.
As part of this enhancement, the comparison options are no longer auto-selected. Users must also now choose which components to exclude, rather than include. By default, all components are included in the comparison.
Enablement & Configuration
This feature is available automatically in Studio, with no additional configuration required.
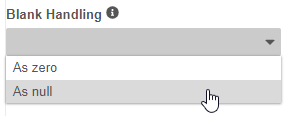
Set Blank Handling in Rule Editor
Use Case
This feature empowers Studio users to configure their Rules independently by removing the need to access Business Admin to edit this setting.
Description
With this release, Studio users can edit the Blank Handling setting for Rules from within the Rule Editor, in the Details section. Prior to this release, administrators were required to set the blank handling on rules from Admin > Business Admin. This setting dictates how Vault handles blank values (for number and unit items) within the rule expression.
Enablement & Configuration
This feature is automatically available in all Studies using version 2 of the expression grammar.
Study Administration
Features in this section apply to EDC Tools, a study-level administration area for Vault EDC.
Temporarily Removing Import & Export for Study Users
Description
With the 19R2.3 release, we have temporarily removed the ability to import and export study Users from EDC Tools. This functionality will return in a later release.
Enablement & Configuration
This change applies automatically to all Limited Release CDMS vaults.
Deployments
Features in this section are enhancements to deployment functionality in Vault CDMS.
Automated Deployments
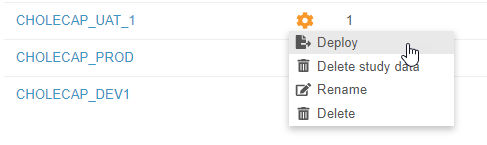
With this release, we introduced automated deployments to Vault CDMS. This enhancement includes a number of cross-application features that allow users to easily manage study environments and deploy their study changes from development to UAT, and ultimately deploy to production, with a single action in the EDC Tools user interface.
Enablement
Existing studies must undergo an upgrade process to use the new deployment model. Contact Veeva Services to discuss enabling this feature in your vault.
Once this feature is enabled in your vault, it applies to new studies immediately. For existing studies, there is some minor default impact, but your study must undergo an upgrade process before using the new deployment model.
Existing Studies
Existing Studies do not automatically move to the new deployment model once it is enabled. Vault only adds a Cross Vault Unique ID field to the Study object, to prepare these Studies for the upgrade into the new model. Studies that are in production and have no pending changes, or studies in development that have not yet been deployed, can both be upgraded.
Users can continue to manually manage their deployments for existing Studies.
New Studies
Once this feature is enabled, all newly created Studies are automatically on the new deployment model. Users can identify study environments upon Study creation in Studio or upon initial deployment.
New Standard Roles
For this feature, we added the new CDMS Deployment Administrator study role, to be used exclusively for deployment of study design and vault-level settings between study environments. To support the profile-based security model, we also added an EDC Deployment Administrator application role. This role has all of the required permissions granted with dynamic access control and a security profile.
We also added a new functional permission, Manage Study Deployments, which grants users the ability to create and edit Study Masters and Study Instances (environments) as well as deploy changes from environment to environment.
The CDMS Deployment Administrator study role has the following permissions:
- EDC Tools Tab
- Manage Study Lock
- Manage Study Deployments
Impact by Application Area
The sections below list the impact of this feature by application area:
Studio
We replaced the New Study dialog to accommodate collecting additional information for creating a Study Master and related environments. We added the ability to navigate from a listing of manual Studies to a listing of automated deployment Study Masters. After selecting a Study Master, users can then select a Study Environment (created by a deployment administrator in EDC Tools) and begin working in Studio. We replaced the breadcrumb in Studio to support navigation between automated and manual deployment studies.
EDC Tools
EDC Tools now opens to a list of Study Masters. Users can click into a Study Master to view the Deployments page, where they can select or create a Study Environment, and then enter the primary EDC Tools area. Deployment administrators can create new Study Environments for development, training, user acceptance testing, and production in a Study Master from this listing. Once a study’s design is complete, a deployment administrator can deploy the design into another Study Environment from this listing as well. Vault tracks those deployments in the Deployment History page. When deploying a study, deployment administrators can choose to delete study data (if deploying from a development, UAT, or training environment) and to export a Detail PDF. We also refreshed the user creation flow in EDC Tools to accommodate both deployment models.
From vaults that are designated as the primary development environment, deployment administrators can see all Study Environments, including production.
Coder Tools
Coding configuration is part of the study package that vault deploys from environment to environment. This includes Coder study settings, the Names of any Synonym Lists, and the Names of any Do Not Autocode Lists (formerly known as Stop Lists). Vault does not deploy the contents of Synonym Lists or Do Not Autocode Lists. Coding administrators can use existing import and export functionality for these lists to move their contents from environment to environment. If a change to coding configuration would change a verbatim term or dictionary version, deployment will fail if there are any open Code Requests that coincide with that configuration. This failure will require redeployment of the Study.
Business Value
The current, manual process to manage study deployments requires multiple, highly manual steps, making each deployment time-consuming and prone to errors. Environments are managed as separate Studies on multiple vaults. To deploy a new casebook version from UAT to production, a user must export it from their UAT vault, log out of their UAT vault, log in to their production vault, and then import that new version, all before they can actually run the amendment.
In this new model, users can create all study environments under a single study, the Study Master, and link those environments and the vaults they reside in together. Users can view and manage these environments from EDC Tools and Studio.
Users can use EDC Tools to manage their deployments. Using a simple set of menu actions, they can deploy changes from one environment to the next. Vault performs all deployment-related steps as part of that one action. This reduces the potential for error and moves a multi-step, manual process into one action.
Feature Enablement Summary
| Feature Name | Enablement | Application |
|---|---|---|
| CDMS | ||
| Audit Trail Enhancements | Auto-on | EDC, Coder |
| Detail PDF Enhancements | Study Feature Flag | EDC |
| Data Review | ||
| Review UI Enhancements | Auto-on | EDC |
| Removed Review Status Propagation | Auto-on | EDC |
| Vault Coder | ||
| Ability to Change the Dictionary for a Form | Auto-on | Coder |
| Coder General Configuration Enhancements | Auto-on | Coder |
| Renamed "Stop Lists" as "Do Not Autocode Lists" | Auto-on | Coder |
| Extend a Coding Decision to All Forms & Update the Synonym List | Auto-on | Coder |
| Dictionary Search Enhancements | Auto-on | Coder |
| Reports & Dashboards | ||
| Track Queries Leading to Data Changes | Auto-on | EDC |
| Enhanced Coder Metrics | Auto-on | EDC |
| Assessments | ||
| Clinical Review Assessments | Support | EDC |
| Study Design & Configuration | ||
| Studio Usability Enhancements | Auto-on | EDC |
| New Studio Navigation Pattern | Auto-on | EDC |
| Compare All Versions in Studio | Auto-on | EDC |
| Compare Forms Across Studies | Auto-on | EDC |
| Set Blank Handling in Rule Editor | Auto-on | EDC |
| Study Administration | ||
| Temporarily Removing Import & Export for Study Users | Auto-on | EDC, Coder |
| Deployments | ||
| Automated Deployments | Support | EDC, Coder |
Enablement Legend
| Enablement | Description |
|---|---|
| Auto-on | Automatically activated and no configuration is required before using the feature; note that in some cases, a new feature is dependent on another feature that must be enabled or configured. |
| Study Feature Flag | This feature is available by configuration within the Study Configuration object (or similar). To enable a feature using study configuration, navigate to Admin > Business Admin > Study Configuration and edit the Study Configuration record for your Study. |
| By Study Build | The configuration options for this feature are available automatically in Studio, EDC Tools, Coder Tools, or System Tools, but you must configure them within your Study for those options to apply. |
| Support | On/off option controlled by Support. |