New Features in 19R1.4
Transfer Subjects, Coder Dashboard, and more...
Pre-Release Date: July 2, 2019 | Release Date: August 2, 2019Pre-Release Date: July 2, 2019 | Release Date: August 2, 2019
We are pleased to bring you the following new features in this week’s release. See details about each feature’s enablement below.
Data Entry
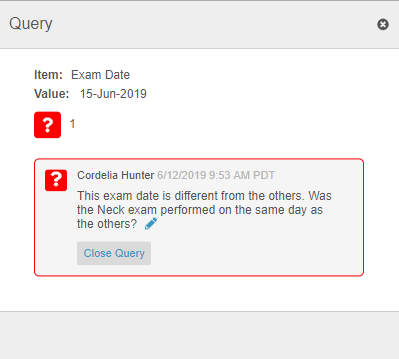
Queries in Repeating Item Group Tabular View
For repeating Item Groups in tabular view, Vault now shows the Open Query and Answered Query icons in the Item value’s table cell. If there are multiple queries with different statuses on the same Item, Vault displays the Open Query icon before the Answered Query icon. Once a user clicks on the query icon, Vault displays all queries on the Item in the new Query dialog, where users can answer queries.
|
|
|
Coder
Dictionary Database
In preparation for future enhancements, we released new infrastructure such that any CDMS application vault or Safety application vault can remotely access MedDRA or WHODrug (B3 and C3) dictionary records, once the customer’s subscription to their dictionary is verified.
Vault can pull dictionary search results directly from the Dictionary Database. Vault can also perform upversioning and dictionary-based autocoding directly from the Dictionary Database.
New Dictionary Search with Enhancements
Now that dictionary records are available in the new Dictionary Database, we built the Dictionary Search algorithms in SQL. In this effort, we also introduced several enhancements. The enhancements include displaying only unique records in the Dictionary Search results, capturing all Preferred Name varieties of the same Drug Name, allowing up to 10 search terms in search phrases, and the ability to search all special characters.
Reports & Dashboards
Vault Coder Dashboard
Vault Coder users can now view high-level metrics for code assignments, coding progress, and query statuses from the new, standard Vault Coder Dashboard.
Studio
In this release, Studio is only available to a limited set of early adopter customers.
Enable Forms for Coding from Studio
A CDMS Study Designer or other Studio user can now identify coding Forms and configure them in Studio so that they are enabled and integrated for coding within Coder. To configure a Form using this feature, a Studio user must identify the Verbatim field, and any other necessary supporting fields, such as Indication, Route, or Seriousness, and assign a dictionary release. They can now also identify which fields Vault will use to inform coding and autocoding (such as Indication and Route for WHODrug) and which fields should inform prioritization (such as Seriousness for MedDRA). They also have the option to assign a Synonym List and Stop List.
ODM Import & Export Includes Coder Study Settings
The ODM XML file in Studio now includes all Coder Study Settings, including the Therapeutic Area. Prior to this release, a coding administrator had to manually define those settings in each vault for a Study. With this enhancement, a coding administrator only needs to define their study-level settings once, and then Vault will automatically apply those settings whenever the study design is imported into another Study or into the same Study in another vault.
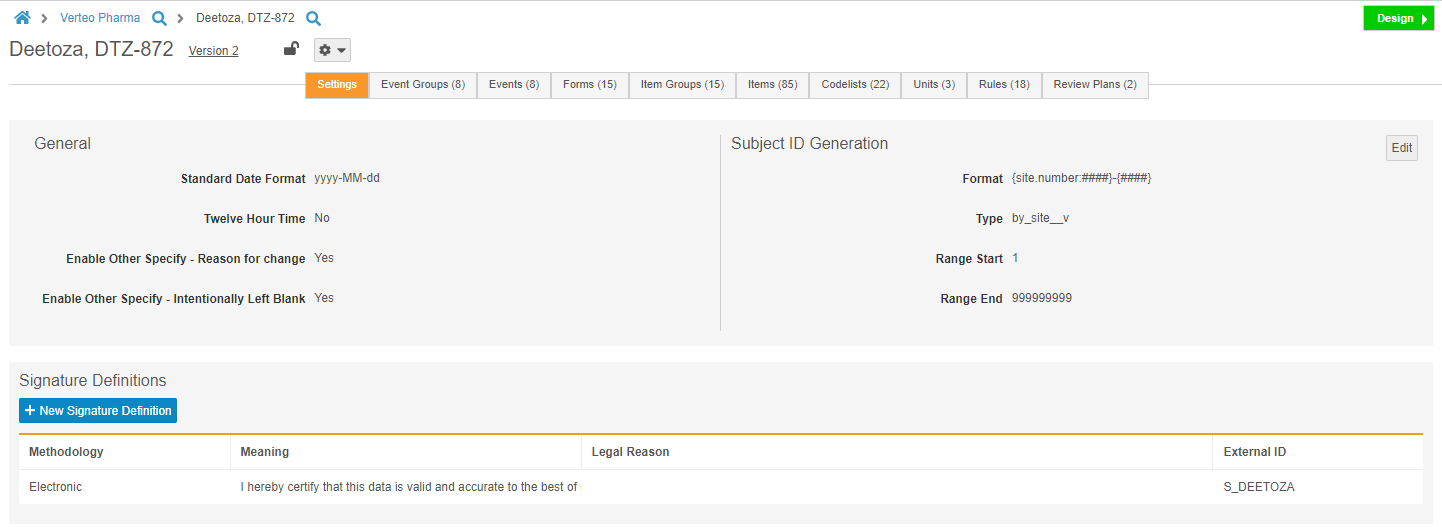
Manage Study Settings in Studio
Studio users can now manage study-level settings, including Subject ID generation settings and the Signature Definition, from the new Settings subtab in Studio. Prior to this release, Admins could only change these settings by editing multiple object records from Admin > Business Admin. This enhancement allows Studio users to manage these settings easily and from a single location.
New Options & Information in the Compare Versions Report
With this release, Studio users can now also select to include Reviews in their comparison report. The comparison report now also includes the new Codelist and Unit and Form Impact worksheets by default. Note that because Review Plans and study settings aren’t versioned, the comparison report only shows differences when comparing different Studies.
| Worksheet | Description |
| Form Impact | Displays a row for each form in the study and a column for each event, with a marker indicating which events contain which forms. |
| Codelist and Unit | Displays all codelist and unit definitions in the study, their items, and their difference status. |
| Reviews | Displays review plan items, grouped by Review Plan, and their difference status. |
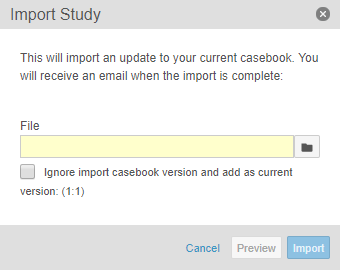
Ignore Casebook Versions in Import File
With this release, users can now import a different Study into their current Study and reset the file’s casebook version to the current casebook version. For example, if the import file contains Version 4 of the Deetoza, UAT study, a study designer can import that file into the production Deetoza study as Version 1 by selecting the Ignore import casebook version and add as current version checkbox.
EDC Tools
Availability: In the current release, this area of the application is only available to Veeva Services.
Audit Trail Export includes Deleted Records
When records are deleted in Vault CDMS, for example, due to a casebook amendment or subject deletion, the Audit Trail Export job, scheduled from EDC Tools > Jobs, now includes those audit trail entries.
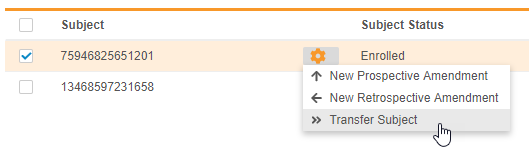
Transfer a Subject to a Different Study Site
Lead data managers can now transfer a Subject from one Site to another Site within the same Study and Study Country, as well as provide a reason for the subject’s transfer. Subject transfer is permanent. Vault does not change the Review Plan assigned to a subject even if the transfer moves the subject to a Site with a different template Review Plan. Lead data managers can update the subject’s assigned Review Plan if needed.
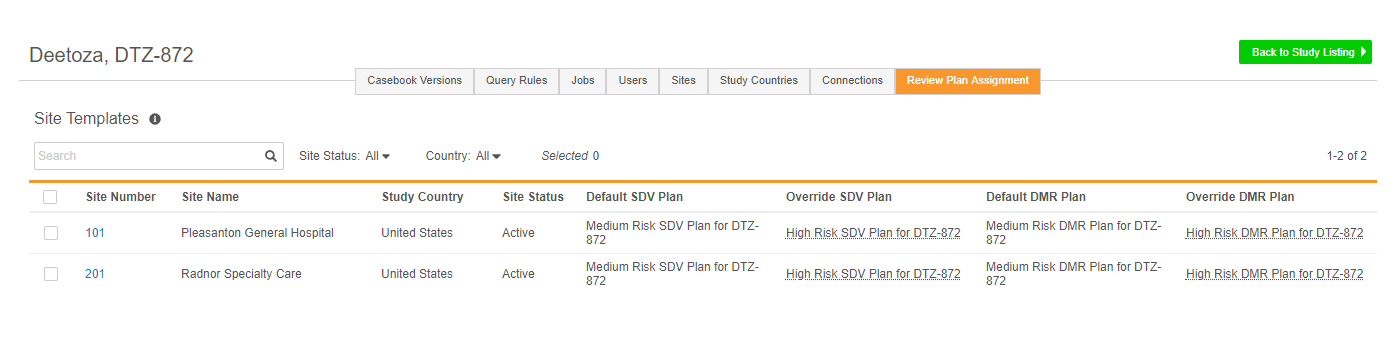
Site Review Plan Assignment
Vault uses Review Plans to apply targeted Source Data Verification (SDV) and Data Management Review (DMR) requirements to data collection forms and items. Different Sites can use different Review Plans. For example, a more mature clinical site where data collection tends to be of higher quality, can have a Review Plan with fewer forms requiring SDV and DMR. A site that is conducting a clinical trial for the first time may need to perform SDV on all of their forms. Once a study designer creates a Review Plan in Studio, a lead data manager can assign it to a Site from EDC Tools.
From EDC Tools > Review Plan Assignment, the lead data manager can define a default Review Plan, which Vault assigns to Sites at creation. Then they can also define an override plan, which Vault applies based on preset subject selection criteria. Users can also manually assign the override plan on a subject-by-subject basis. Users can also change a site’s assigned Review Plan at any time during the study and run the SDV or DMR re-assignment job to apply the new plan retrospectively to all Subjects at a Site.
Retrospective Amendment & Study Update Restriction Changes
With this release, we updated the allowed changes between casebook versions and during retrospective amendments.
The following changes are now allowed:
- Changes to the event window
- Increasing or decreasing the Repeat Maximum of a repeating Event Group, Form, or Item Group
The following changes are now not allowed:
- Changes to a design definition record’s Name
- Updating the Allow Unknowns selection to an option that allows fewer unknowns than the previous (for example, you can’t switch from Date and Time to Time)
Security
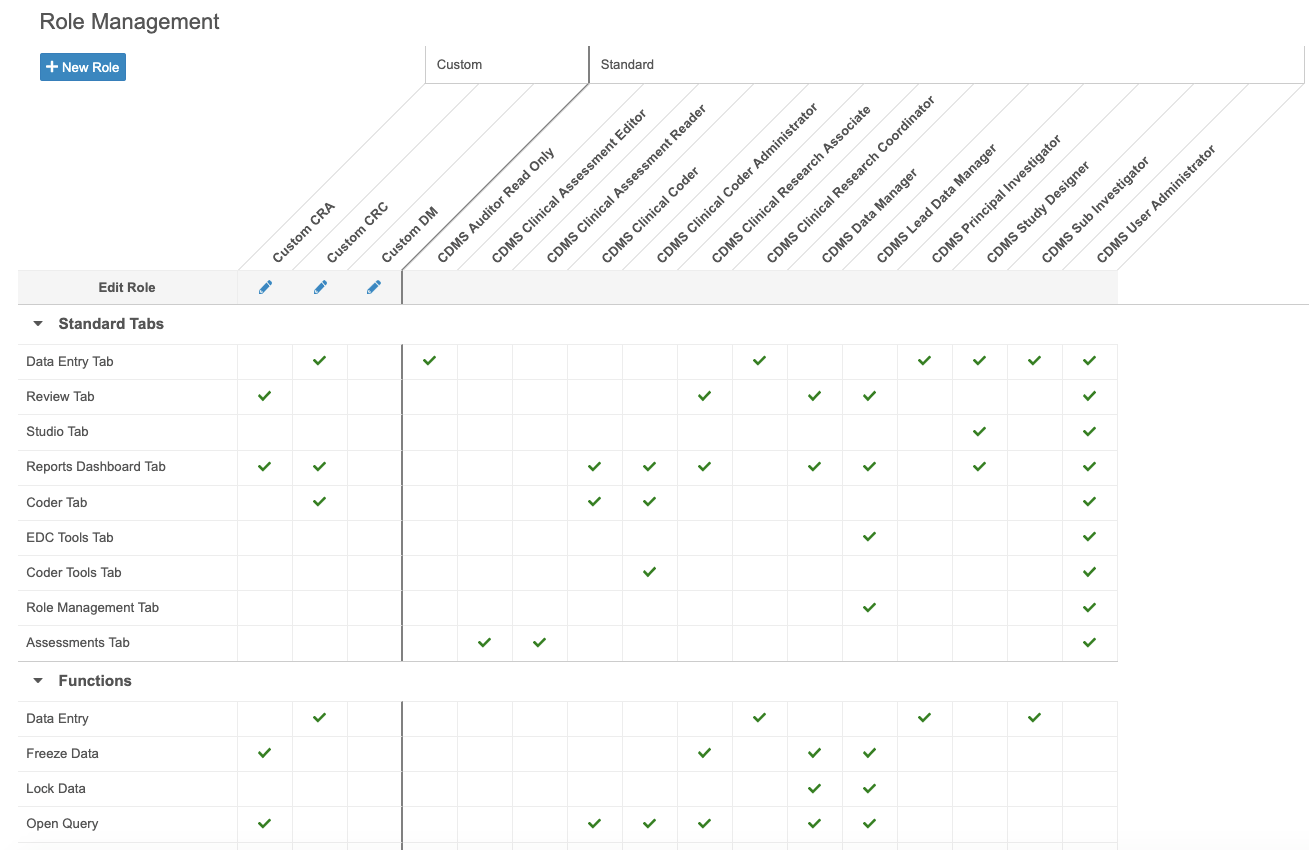
Custom Role Management
With this release, CDMS Lead Data Managers and CDMS User Administrators can now easily create new, custom Study Roles (formerly labeled as Application Roles), as well as copy existing and standard Study Roles, from the new Tools > Role Management area. They can also view existing custom and standard Study Roles, and the functional permissions associated with each, from this area. A Study Role represents a group of functional permissions, combining Application Roles and Security Profiles into a single entity.
As part of this feature, lead data managers and user administrators now only select a Study Role when creating a new User from EDC Tools > Users, instead of selecting an Application Role and a Security Profile.
In this release, Vault only supports custom Study Roles with standard tabs and functional permissions. Custom objects and tabs are not supported in this release.
Feature Enablement
| Feature Name | Enablement | Application |
|---|---|---|
| Data Entry | ||
| Queries in Repeating Item Group Tabular View | Auto-on 1 | EDC |
| Coder | ||
| New Dictionary Search & Enhancements | Support | Coder |
| Dictionary Database | Support | Coder |
| Reports & Dashboards | ||
| Vault Coder Dashboard | Support | Coder |
| Studio | ||
| Limited Availability: In the current release, this area of the application is only available to Studio early adopter customers. | ||
| Enable Forms for Coding from Studio | Auto-on | Coder, EDC |
| ODM Import & Export Includes Coder Study Settings | Auto-on | Coder, EDC |
| Manage Study Settings in Studio | Auto-on | EDC |
| New Options & Information in the Compare Versions Report | Auto-on | EDC |
| Ignore Casebook Versions in Import File | Auto-on | EDC |
| EDC Tools | ||
| Internal: In the current release, this area of the application is available only to Veeva Services. | ||
| Audit Trail Export Includes Deleted Records | Auto-on | EDC |
| Transfer a Subject to a Different Site | Auto-on | EDC |
| Site Review Plan Assignment | Configuration | EDC |
| Retrospective Amendment & Study Update Restriction Changes | Auto-on | EDC |
| Security | ||
| Internal: In the current release, this area of the application is available only to Veeva Services. | ||
| Custom Role Management | Auto-on 2 | Coder, EDC |
|
Enablement Notes 1 In repeating Item Groups configured to use Tabular View 2 In vaults where Role by Study is enabled |
||
Enablement Legend
See the following explanations for feature enablement options:
| Enablement | Description |
|---|---|
| Auto-on | Automatically activated and no configuration is required before using the feature; note that in some cases, a new feature is dependent on another feature that must be enabled or configured. |
| Admin Checkbox | Admins must turn on the feature with an Admin checkbox. Note that some “Auto-On” features have a checkbox setting that hides the feature; these will show “Auto-On.” |
| Configuration | Admins must configure the feature (separately from an Admin checkbox) before it is available to use or is active; for example, a Clinical Programmer must create an Item Definition of a certain new Item Type. |
| Support | On/off option controlled by Support. |