What's New in 17R3
| Pre-Release Date: November 6, 2017 | Release Date: December 8, 2017 (EDC on December 15, 2017) |
We are pleased to bring you Vault 17R3. Read about the new features below. You can find information on enabling new features in 17R3 Feature Enablement Details.
AWS Migration
As part of our commitment to ongoing innovation and customer success, Veeva is moving its worldwide computing infrastructure from managed data centers to the Veeva virtual private cloud (VPC) running on Amazon Web Services (AWS). This began in the second half of 2017 across all Veeva product lines.
EDC
Links to Item Groups in Repeating Form Tables
In EDC, a study design can include repeating Forms with repeating Item Groups. When a user views a repeating form in the table view, they can see one column for each repeating item group on that form. Each row in a repeating Item Group’s column contains a link to view the repeating group on its form.
Prevent Auto-Creation of Initial Repeating Form Record
When a repeating Form is first added to a Casebook, Vault will no longer automatically create an initial repeating Form record. Repeating Forms may not be required or used during a study. This feature eliminates the work of removing unused repeating Form records. A data entry user can click + New to create the first repeating Form.
Reference Number for Repeating Forms
Repeating Forms are automatically assigned a reference number that displays in both form and table view, as well as in the form carousel. This number is now labeled as Reference #.
Targeted SDV
With the introduction of Targeted SDV, a study sponsor can define SDV requirements for a subject in a Review Plan. Targeted SDV is extremely flexible and supports varying SDV requirements across all Items at the Event-_level. Sponsors can also configure Targeted SDV for some _Events to be very granular, drilling down to a specific Event, Form, Item Group, and Item combination. For each Item in the Targeted SDV Review Plan, there are three (3) modes available: Required, Optional, and Not Available. When an Item is Not Available, Vault EDC does not display an SDV checkbox for that Item in the UI. Sponsors can configure Targeted SDV plans at the Study-_level for all _Sites in that Study. Targeted SDV empowers sponsors to better define their review plans. Because clinical monitors now only need to SDV Items where it is required, those users can save time.
Default Permission Changes for SDR
With the 17R3 release, source data review (SDR) permissions are no longer included by default in EDC security profiles. If SDR functionality is required, an Admin must set up SDR permissions for appropriate user security profiles. This change does not affect existing SDR functionality.
Note that this change will not impact existing vaults and users.
Detail PDFs
In Vault EDC, users can export subject Casebooks and individual Forms to PDF to print or use as a reference. With this feature, users can export detailed PDFs, including both Item values and audit trail entries, at the Form- and Casebook-levels. Users can also choose to include queries in their PDF.
Icon for Intentionally Left Blank Form
Currently, data entry users can mark an entire Form as Intentionally Left Blank when data is not available. After a user marks a Form as Intentionally Left Blank, other users can now save time and distinguish between a Submitted Form and an Intentionally Left Blank Form with a new status icon for Intentionally Left Blank.
Updated Icons for Form Status
In this release, we updated the icons for Form status to be more visually consistent across different statuses. There is no change in behavior for which icons display on the casebook schedule. Each status still corresponds to an icon, and the icon only displays when a Form is in a given status.
Task Bar Performance Improvements
In this release, we updated the task bar’s count feature to improve performance. For users with access to multiple Studies, the task bar does not display any task counts until the user selects a single Study. After Study selection, the task bar counts display, and users can click into each type of tasks as they were able to previously.
Skip Item (Dynamic Entry)
Based on study design, specific Item controls can be initially disabled until certain criteria are met in controlling Items. For example, a Pregnancy Test Result field remains disabled until a user selects Female for Gender. The disabled fields are visible and displayed to data entry users, but they cannot enter data in the Item until the rule criteria are met. If a user later edits a controlling Item so that rule criteria are no longer valid, the dependent Item remains enabled, and any data or open queries remain enabled as well. Once the data is cleared or the query is closed, and a user submits the Form, the dependent Item is once again disabled. After that, if user edits the Form, dependent Items remain disabled for data entry. This feature saves a great deal of time for both data entry users and monitoring users. When a field is not required or irrelevant based on entered data, data entry users no longer need to mark an Item as not available, and monitoring users no longer need to SDV or SDR irrelevant data.
Dynamic Event Groups
In order to support multiple study designs, for example, randomized or adaptive studies, Clinical Programmers can create data validation rules to dynamically add Event Groups to subject casebooks based on conditions. When subject data meets specific rule criteria, Vault EDC automatically adds the Event Group to the subject’s casebook. For example, in a multi-arm Study, Vault EDC can dynamically add the appropriate study arm Event Groups for a subject based on their randomization number. Dynamic Event Groups ensure that data entry users are only presented with the necessary Events and Forms.
Sort the Casebook Schedule by Scheduled or Actual Date
On the casebook schedule, EDC users can choose to sort events in two different ways. Users can view the default casebook schedule with Events ordered by the Study schedule, and with unscheduled events at the bottom. Users can also toggle to sort Events sorted based on actual Event date recorded for the Events.
Changing an Event Date Prompts Reason for Change
When users change Event’s dates, Vault prompts them to select a reason for change in the same way as when they change Item values. Organizations can work with Veeva Support to configure the list of reasons for change to have different change reason options than Item-level change reasons.
Freeze & Lock all Forms in an Event
This feature allows EDC users with freeze or lock permissions to be able to apply or undo these actions to all forms in an event. Users can apply these actions by selecting Freeze All Forms or Lock All Forms from the Event’s More Actions menu.
Event Queries
Users with the ability to open manual queries can now create queries against an Event. Users can use Event queries to raise questions or get clarification about Event-level details, such as the Event date. To create an Event query, users with Create Query permission (CRAs and data managers) can select Open Query from the Event’s More Actions menu.
Users who answer queries (Investigators and CRCs) can do so from the casebook schedule, in the same way they would for an Item query.
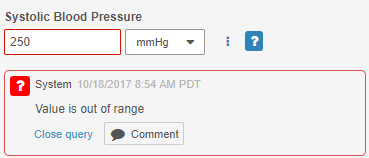
System-Generated Query UI Update
On system-generated Queries, the Reply button is now labeled Comment, and the color of the button is now gray. This label and styling change emphasizes that this button is for replying to a comment, rather than updating a queried Item value.
Icon Badge for Queries
Instead of a separate Open Queries status icon, queries are now represented by a badge icon on top of a Form or Event status icon. With this feature, users can simultaneously see the current Form or Event status and identify if it has open queries.
Standard Report Templates & Report Types
With this release, Vault EDC includes standard report templates and report types. These reports provide a reference for custom reports that customers may create during the conduct of their study, so that end users may draw study- and site-level insights to improve study execution. Template reports and report types are Vault EDC’s recommended display of study operational metrics. Customers can create new reports, or copy and edit standard report templates, as needed during the execution of their study.
EDC Study Administration
Study Administration
With this release, Vault EDC includes a dedicated Study Administration area. Study managers, or other users with access to all Study Sites can access this UI by clicking the Study Tools button. In the study tools area, study managers manage their casebook versions, without having to access the Vault Admin area or use Studio.
Casebook Version Management
In the study tools area, a study manager can easily view the available Casebook Definition versions for their Study and the number of existing subjects using each version. Study managers can activate a Study Site to use a specific casebook version upon new Casebook creation. This feature allows one site to use a different casebook version, while other sites might be on later versions. Casebook version management in study tools is especially helpful to study managers during IRB approval of Forms and casebook versions.
EDC Studio
Study Versioning
Study administrators can control and build new Casebook Definition versions for a Study. This feature includes the ability to lock casebook versions to prevent changes and create new versions for editing. With versioning, study administrators and clinical programmers can export and import versioned Casebook Definitions. This feature supports study design changes without impacting existing Casebooks.
Definition Objects Read-Only when Published: After an Admin publishes the Study, all definition objects (except for Rules) become Read-Only. This feature provides a mechanism to control inadvertent changes to the study design outside of an expected study update.
Object Reuse in Studio
With object reuse, clinical programmers can manage and reuse standardized object definitions in a single Study and across multiple Studies in Studio. Clinical programmers can validate standards, make references to other objects, and override Labels to allow the use of objects as templates for other objects. Clinical programmers can also copy Unit Definitions and Codelist Definitions for reuse from the Properties panel. Clinical programmers can also copy Item Definitions, Unit Definitions, and Codelist Definitions for reuse in a single Study from the Properties panel. With this feature, clinical programmers can develop a library-like collection of study design object definitions in Studio, saving time and increasing consistency.
System Rule Management in Studio
With this release, users can manage future date, mandatory (required), and range fields from an Item’s Properties panel, rather than through Admin > Business Admin. Users can also import and export system rules during casebook versioning. This provides the ability to maintain these data validation rules in Studio, simplifying the study development process and saving clinical programmers time.
timeDiff Function for Data Validation Rules
With this release, clinical programmers can build rules to return the difference between two times, using the function timeDiff(time1, time2). When clinical programmers build rules using this function, Vault EDC can validate that a given DateTime– or Time-_type _Item value falls within a given range, further automating the data validation process. Learn more about building rules with the timeDiff function.
Insert Identifiers into Rule Expressions
With this release, users can select click Insert Identifier when creating rule expression. Instead of typing up to four identifiers, users can select the Event, Form, Item Group, and Item in a dialog. Vault then inserts a properly formatted identifier at the cursor location. In addition, the Criteria field is now labeled Expression for clarity. This feature saves users time, and it increases accuracy and consistency in rule expressions.
Skip Item Rules
Clinical programmers can build new Rules to enable and disable Items on a Form. By building Rules with the Skip Item action. This feature allows users to conditionally enable Items in EDC for entry based on answers to previous Items. Users can enable or disable multiple Items with a single Rule. This feature supports Skip Item (Dynamic Entry), which saves data entry and monitoring users a great deal of time. Learn more about building skip item rules.
Cross-Form & Cross-Event Data Validation Rules
Clinical programmers can build data validation rules that evaluate data across multiple Forms or Events. Clinical programmers can use identifiers for Items in multiple Item Groups, Forms, and Events in rule expressions. Identifiers must include a full path for specific hierarchies or an implied path for Vault to process the rule. For example, a clinical programmer could build a rule that creates a Query when Systolic and Diastolic Blood Pressure on a Vitals form are higher than in an earlier Event and Form.
Collapse & Expand Components in Design View
This feature allows users to collapse and expand all components in the Design view. Users can more easily navigate large visit schedules and form designs with less scrolling. Users are still able expand and collapse objects at an individual object level.
Search Object Definitions
EDC Studio users can now search in an object definition tab in Browse view. Users can search using any property value, except for the Label and Short Label. This enhancement saves clinical programmers time by allowing them to search for and view only relevant records, instead of paging through to locate the object definition they need.
Vault Objects
Pass Context into Create & Relate
Vault can simplify data entry processes for users by automatically filling in data. This feature streamlines the create and relate object records process for certain objects. In vaults where two objects share the same parent object and have a relationship to each other, Vault auto-populates the parent object field when using Create to create a record for a sibling object from the context of the related record.
For example, in RIM vaults, Submission and Regulatory Objective objects share the Application parent object. When creating a related Submission record from a Regulatory Objective record, Vault auto-fills the parent object field to use the same Application record as the Regulatory Objective uses.
List Navigation for Related Items
In past releases, users could page through a list of related records in most situations, but not for records in a complex many-to-many relationship. This release expands the navigation functionality to support this situation.
Inline Edit of Related Items
Inline grid editing simplifies data entry by allowing users to edit directly in a list. In prior releases, the feature was available within object tabs. This release extends inline edit to related lists that appear with object detail pages. As users update individual cells within the list, Vault validates and saves their changes.
Additionally, users can now access the same customization options for related record sections as they use for object list pages:
- Change the visible columns to show the most relevant data by choosing Edit Columns from the Actions menu.
- Drag columns to rearrange.
Object Field Defaults
With the Object Field Defaults feature, Admins can set default values for object fields to populate upon object record creation or save. These defaults can use a literal value, such as a specific number, or a formula. Formulas for field defaults use functions and operators to combine literal values and tokens. Users can override default values if they have permission to edit the field. By using object field defaults, organizations can ensure consistency across fields such as Serial Number or that a field, like Legal Question, is always set to Yes on certain records.
Collapsible Page Sections
-
All sections except the first details section appear in a “collapsed” state by default.
-
Users can expand/collapse individual sections by clicking on the section heading.
-
Jumping to a section using the Navigation Panel on the right of the page automatically expands that section.
With this feature, we’ve also changed how related records and documents display within a section. In past releases, each section showed up to four (4) items by default and allowed users to expand to show up to 25 items. In this release, we show 20 items at a time and allow users to page through the items.
Page Links
Admins can create page link URLs that navigate users to custom page destinations, such as a custom user interface for simplifying data entry into an object for tracking events. At runtime, page links direct users to the URL defined by the link as opposed to the standard object record detail page. Admins can create page links with custom URL destinations for the following object record actions:
- Create
- View
- Edit
- Copy
Page link URLs can be internal or external. Vault loads external pages in an iFrame.
Object Record Permission Details (UI Enhancement)
In this release, we’ve modified the UI to allow Admins to edit object permissions at the profile and field level from the Users & Groups > Permission Sets > [Permission Set] > Objects > [Object] details page. This update uses the same design as Vault’s object page layout providing consistency and improved usability for users.
System-Managed Name Enhancements
Today, Admins can configure Vault to automatically generate object record names by enabling the System manages field value for the Name field. This feature enhances the Value Format to support field tokens, in addition to the literal strings and auto-numbering tokens supported today.
Field tokens can come from the same object or from objects related through outbound relationships. Vault supports tokens for the following field types:
- Text
- Number
- Picklist (single-value only)
With this release, Admins can also change the Value Format and Starting Number for standard objects with System manages field value enabled..
Object Reference Constraints Enhancement
Business Admins can configure object reference constraints based on Lifecycle State. This allows users to view and select from a list of different reference records in different lifecycle states.
Lifecycles & Workflows
Workflow Task Due Dates Based on Object Fields
When configuring object workflows, Admins can now set task due dates based on date field values from the object record, with or without an offset value. This enhancement simplifies data entry and reduces the chance of users entering dates incorrectly by leveraging values that already exist on the object record. For example, an organization that needs to review Product records every six (6) months could set due dates based on Last Product Review Date plus 180 days.
Vault Loader & Configuration Migration
Vault Loader Success & Failure Log Enhancements
Vault Loader now creates separate logs for successful and failed uploads. Failure logs contain the original data along with the corresponding errors so users can easily review and fix errors. After correcting errors, users can import the failure log itself, rather than updating the original file for import.
Vault Configuration Report
Vault Configuration Report allows a user to extract the configuration of a vault into an Excel report. This report includes configuration of components as well as reference data used by the components. The Vault Configuration Report is particularly useful for tracking a vault’s configuration during migrations and implementations.
Vault Compare Permission
Admins can now grant or limit access to the Vault Compare feature using custom security profiles and the new Environment: Vault Comparison permission.
New Supported Component Types
The following component types are now supported for Configuration Migration Packages, Vault Compare, and Vault Configuration Report:
- Document Matching Rule (sharing rules for Dynamic Access Control)
- Jobs
- Report & Dashboard
- Rendition Type
- Workflow Participant Rule
- Document Relationship Type
- Field Layout
- Formatted Output Template
Vault Java SDK
Debug Log for Vault Java SDK
Vault Java SDK allows Veeva Services to program custom business logic which executes whenever users perform an operation on records. With the addition of the Debug Log, programmers can easily troubleshoot issues in their code. The Debug Log captures details about programming errors and errors which result from exceeding governance limits (Vault Java SDK errors). To inquire about Vault Java SDK solutions, contact Veeva Services.
Authentication
Register OAuth2/OIDC as Metadata in SSO Profile
To enable external applications to discover and configure the login requirements and authentication flows for Vault OAuth2.0 / OpenID Connect users, those applications need to obtain the remote authorization server metadata. This feature allows configuration of remote authorization server metadata so it can be discovered and downloaded by external applications.
Google Chrome Blocks Saved Passwords in eSignature
To provide better security, Veeva blocks most browsers from remembering passwords. A recent update to the Google Chrome browser allowed it to remember Vault passwords in some circumstances. With this release, Vault has modified the eSignature password field to prevent Chrome from remembering passwords.
As part of this change, some password managers may not auto-fill user credentials properly. We recommend that users enter their passwords manually to avoid becoming locked out because of too many failed login attempts.
Security
eSignature Access for External Users
In past releases, users with the External User license type did not have access to provide eSignatures by default. Admins were able to allow this by assigning the users to custom Security Profiles, but doing so triggered license violation emails.
In 17R3, we’ve lifted this restriction. The standard External User security profile and permission set now provide the permission Workflow > eSignature and Vault does not generate license violation messages.
Vault Java SDK Permissions
In this release we’ve introduced four (4) new permissions: Vault Java SDK: Read, Create, Edit, Delete. Although Java SDK is not yet available, these permissions will appear in your vault. To inquire about Vault Java SDK solutions, contact Veeva Services.
Action Level Security (ALS)
Vault Actions are configured custom actions in Vault that can be executed on an object record. Action Level Security (ALS) provides a unified way to secure those actions. With ALS, you can configure permissions at the Security Profile level to apply to actions across all states, and at the Atomic Security level to provide more granular control by state and role.
ALS: Object Actions
With this release, your organization can work with Veeva Services to define actions for one or more objects allowing users to perform actions on those object records. Objects now have a new Actions tab that displays a list of securable custom object actions. Custom actions are configurable instances of a Vault Action.
For example, the Monitoring Event object in Clinical Operations vaults has a set of actions that are already available to it. However, an organization may want to add a custom action to create a Monitoring Event Survey for a Monitoring Event record. Once Veeva Services configures the custom action, an Admin can apply it to the Monitoring Event object from the Actions tab. This enables users to execute the action directly from a Monitoring Event record. Additionally, an Admin can secure the action using ALS to determine if the action is hidden, view-only, or executable at runtime.
Note that Admins must contact Veeva Services to configure Vault Actions at this time. Additionally, Standard Actions will not appear in the Actions tab at this time.
ALS: Profile Security for Actions
When you configure object actions, you can secure those actions on the User Profile level through permissions sets.
For any object with one or more object actions enabled, Vault displays the Object Action Permission section on an object record’s Permissions page. This section allows you to assign View and Execute permissions on the action.
Vault hides any actions without any set permissions at the User Profile level from users assigned to the profile. Actions with the View permission appear in the Action menu of an object record, but users cannot execute them. The Execute permission allows users to view and execute an action.
ALS: Atomic Security for Actions
With Atomic Security for Actions, you can secure object actions and lifecycle user actions at the role level. This allows you to control which object actions or lifecycle user actions are hidden, viewable (visible but grayed out), or executable (visible and clickable) for a specific role.
Security settings for a given action can vary by lifecycle state, and similar to Atomic Security for object fields, you can override the defaults for certain application roles. For example, an organization’s Quality Vault using the Quality Event lifecycle may apply the View permission to the Define and Execute Changes action while in the Change Plan Approved state. This would make the action visible to all roles, but grayed out. However, the organization only wants the Approver to execute this action. To do this, they would apply the Execute permission to the Approver role.
Reporting
Flash Report Preview
When scheduling a Flash Report, users can select the Include report in email checkbox. With this option, users can easily access and preview reports from the Flash Report email. This option adds an HTML format copy of the report into the Flash Report’s email along with a link to the report in Vault.
Reorder Report Objects
When using report types that include multiple “up” objects, users can now control the order of objects. Users can access this option through the existing Edit Fields dialog. Prior to this feature, Vault always displayed the primary object in the leftmost columns and sorted other “up” objects alphabetically.
This feature also allows users to sort report results on any field from an up object that the primary object references. For example, in a report showing marketing campaigns with documents, users could sort by the Product Approval Date, a field that belongs to the Product object referenced by the Marketing Campaign object.