Exporting Casebook Data as a PDF
In Veeva EDC, you can export your subject’s Casebook to a PDF to print or to use as a reference.
Prerequisites
Users with a standard Application Role and Security Profile can export PDFs from the areas of the application that they have access to (Investigators from the Data Entry tab, CRAs from the Review tab, etc.). CDMS Clinical Coders, CDMS Clinical Coder Administrators, and CDMS User Administrators do not have access to export PDFs. If your vault uses custom Application Roles, your role must have the following permissions:
| Type | Permission Label | Controls |
|---|---|---|
| Functional Permission | Generate Blank PDF | Ability to export blank PDFs |
| Functional Permission | Generate Detail PDF | Ability to export detail PDFs |
If your Study contains restricted data, you must have the Restricted Data Access permission to view it.
Learn more about Study Roles.
Available PDFs
You can export two (2) different types of PDFs for Forms and Casebooks in your Study.
- Blank PDFs that have no data.
- Detail PDFs that have all entered study data.
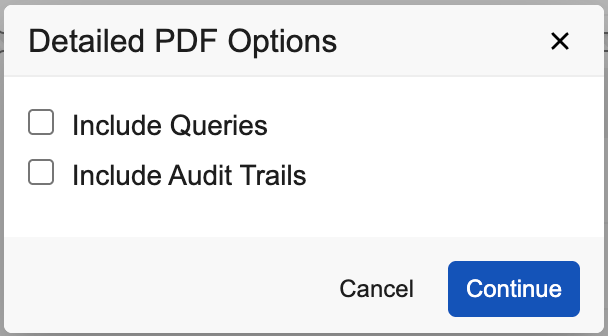
For Detail PDFs, you can choose whether to include queries, audit trail information, or both.
At the Form-level, the PDF includes the Form, as well as the name of the Study, Event, and Form in a header. If you exported a Detail PDF with queries, the PDF also includes sections for audit information and queries.
At the Casebook-level, the PDF includes all Forms in the Casebook, ordered by Event, as well as Study and Subject information in the header. The Event and Form records inside the casebook are bookmarked in the PDF with an option to view By Visit or By Form through the enhanced Detail PDF feature.
PDF Language: Detail and Blank PDFs are available in the language that is set on your user profile. PDFs are translatable, however, only standard text will be translated. Configured labels or entered text will not be translated.
Detail PDF
If your Study was created before the 19R3 release, you may need to enable this feature in order to use Detail PDFs.
File Size Limit: Detail PDFs are limited to 4GB in size.
How to Export a PDF
To export a PDF:
- Navigate to the Casebook or Form you want to generate a PDF from.
- Optional: To export a Subject-level PDF including all Forms associated with the Subject’s Casebook, next to the Subject’s name, click the More Actions menu.
- Optional: To export a PDF of a single Form, navigate to the Form and click the More Actions menu next to the Form name.
- Optional: Click Export Blank PDF to export only Forms without data.
- Optional: Click Detail PDF to export Forms including associated data. The Detailed PDF Options dialog opens.
- Optional: For Detail PDF, select Include Queries to include all associated queries.
- Optional: For Detail PDF, select Include Audit Trails to include audit trail descriptions associated with data changes.
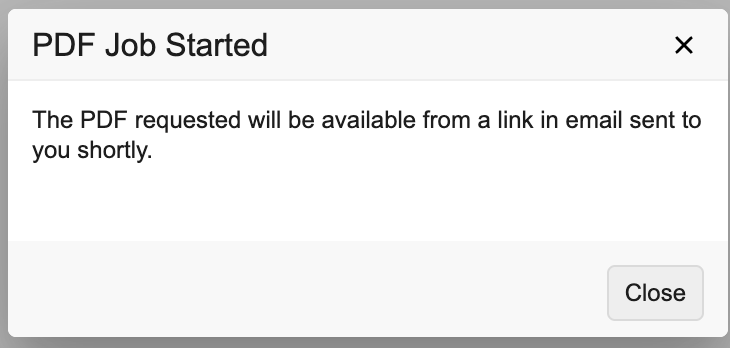
- Vault begins generating your PDF. The system automatically downloads Form-level PDFs. When generation of Subject-level PDFs is finished, you will receive an email with a link to download the PDF.
Export a PDF for All Subjects in a Site
You can export a Detail PDF or a Detail PDF with queries from the EDC Tools > Jobs tab. You can choose to export PDFs for a single Site, a selection of Sites, or all Sites in your Study (closeout PDF). Note that this area of the application is only visible to users with the EDC: Study Tools: Access permission. See Managing Jobs in EDC Tools for details.
Deleted Data in PDFs
If you select Include Audit Trail when generating your PDF, your PDF files include deleted data in the Deleted Data Audit Trail section of the file’s appendix. This includes any data that was deleted as part of the following processes:
- Retrospective amendment with destructive design changes
- Site reset an event or form which was marked for removal
- Site reset a repeating event
- Site removed row in a repeating item group
- Site removed the form linking by deleting a linked form
- Query deletions, undo action in Quick Queries*
- Unscheduled Event is deleted
* The Undo action in Quick Queries is captured as a delete action. It’s included in the audit trail for the queried Item or Event. It’s only included in the Deleted Data Audit Trail section if the queried Item or Event is also deleted.
Only users with access to restricted forms (users whose study role grants the Restricted Data Access permission) will see the Deleted Data Audit Trail. If the user who generated the file doesn’t have restricted data access, and there are deletions of restricted data, then Vault includes a note at the end of the PDF:
Note: The Deleted Data Audit Trail was not included in this file because the user who generated it did not have restricted data access. To include this data, please generate the file again as a user with restricted data access.
PDF Bookmarks
PDF exports include bookmarks to simplify navigation. Blank PDFs include bookmarks grouping Forms within the Events and Event Groups they are part of. The following bookmarks are included for Detail PDFs, depending on which options were selected for the export:
- By Visit: All subject events ordered by study design and containing all forms corresponding to that event.
- Audit Trail By Visit: Audit trail for all subject events ordered by study design.
- By Form: Organizes all Forms alphabetically by Form name.
- Audit Trail by Form: Audit trail for all subject Forms organized alphabetically by Form name.
- Deleted Data Audit Trail: Audit trail of data that was deleted, in event schedule order.
- By Query: Queries ordered by study design.
- Randomization: Randomization information.
Replace Non-Permitted Characters from PDF File & Folder Names
Vault removes non-permitted characters from file and folder names in your PDF exports and replaces them with an underscore (_).
Your organization can choose to instead replace these characters with a hyphen (-).
Enablement: If your vault was created prior to the 25R2 release (August 2025), you must contact Veeva Support to use hyphens instead of underscores. If your vault was created after the 25R2 release, your vault will use hyphens to replace non-permitted characters by default.
When generating the PDFs, any of the following non-permitted characters will be replaced with a hyphen or underscore, depending on configuration:
- Periods (.)
- Whitespaces
- Exclamation points (!)
- At signs (@)
- Pound signs (#)
- Dollar signs ($)
- Carets (^)
- Percent signs (%)
- Asterisks (*)
- Commas (,)
-
Pipes ( ) - Plus signs (+)
- Quotation marks (“)
- Tildes (~)
- Less than or greater than, angle brackets (< or >)
- Curly brackets ({ or })
- Forward or back slashes (/ or )
- Equals sign (=)
- Brackets ([ or ])
- Underscores (_), if configured to replace with a hyphen
When deploying a study, if the user chooses to include Detail PDFs in the deployment file, the file names will not include the replacement hyphens. These files are for internal record keeping and not for regulatory review.