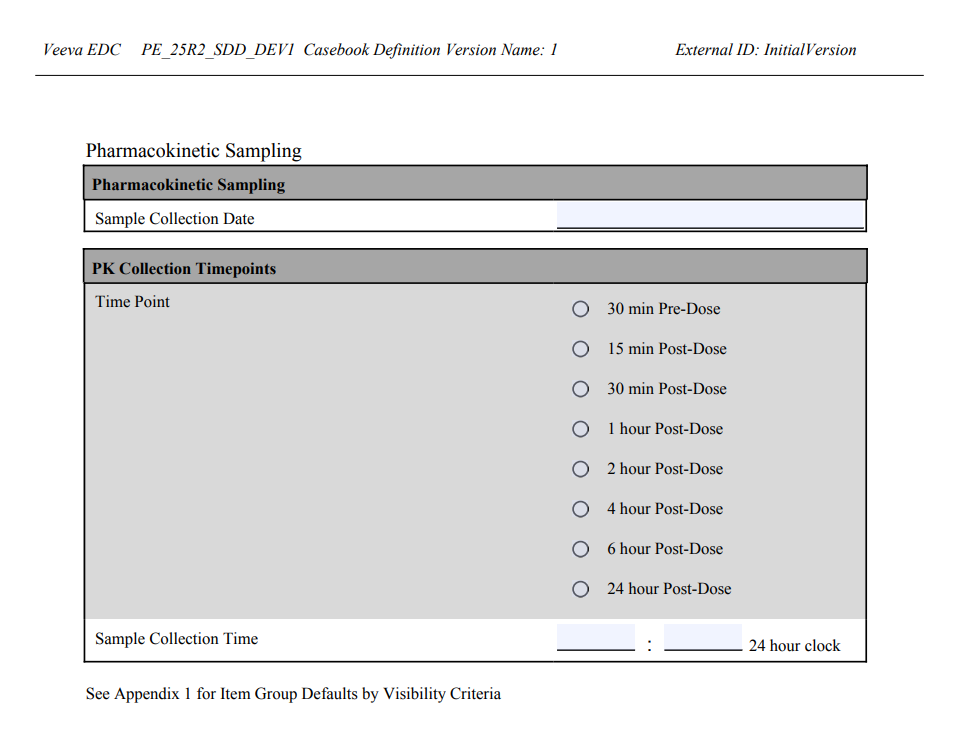
Creating Study Specifications
With Veeva EDC Studio, you can easily generate study documentation at any point in the design process. In the current release, there are four types of study specifications available: an All CRF PDF, a Unique CRF PDF, an SDS, or Study Design Specification (Excel™), and a CDE (Casebook Design Export).
All & Unique CRFs
You can create a PDF file containing all case report forms, or CRFs, in your study. You can choose to include annotations during creation. Annotations include additional detail about item fields on the form, such as coded values, maximum lengths, or if an item field is required.
The PDFs generated for both All CRF PDFs and Unique CRF PDF mirror the blank detail PDFs, available in other areas of the EDC application.
There are two types of PDFs available:
- All CRF PDF: Forms are ordered by their appearance in the study schedule and repeat for each Event they occur in within the schedule.
- Unique CRF PDF: Forms are ordered alphabetically and do not repeat even if they are used more than once in the study schedule.
Annotations
You can choose to include General Annotations and Export Annotations (if available) in your PDF. You can also choose to Indent Progressive Display Items.
General Annotation and Repeating Item Group Display: When you include General Annotations, you cannot select Single Repeating Item Group Copy to create a single copy of repeating Item Groups. When Include General annotations is selected, Repeating Item Groups are shown as a single Item Group with either all Codelist Items or only default Codelist Items depending on the selections for Item Group Default Display.
General Annotations
In the current release, Vault adds the annotations described in the table below. Note that values surrounded with curly brackets (for example, {minimum value}) are tokens and will be replaced with the actual value from your study design.
| Annotation | Description |
|---|---|
| External ID= | Displays the External ID (formerly known as OID) value for each design component. |
| Codelists: | Each Codelist Item annotation includes the coded value for that option. This indicates the stored value that rules evaluate against. |
| Codelist Style: Radio Buttons - Horizontal | Displays when the codelist-type Item uses horizontal radio buttons. |
| Codelist Style: Radio Buttons - Vertical | Displays when the codelist-type Item uses vertical radio buttons. |
| Max Length: | The Maximum Length set for a text-type Item field. |
| Max Length.Precision | The Maximum Length and Precision set for number- and unit-type Item fields. |
| Read Only | This annotation displays on any Read Only type Items. |
| Related Item for Coding | This annotation displays on any Items marked as Related Items for a coding verbatim. |
| Restricted | Displays Yes or No, depending on whether a Form is restricted (blinded). This is based on the Restricted property of the Form Definition. Learn more about Restricted Form data. |
| Required | This annotation displays on any Items that have the Required property checkbox selected. |
| Range, {minimum value} - {maximum value} | This annotation displays on any Items with both minimum and maximum range properties set. |
| Range must be >= {minimum value} | This annotation displays on any Items with a Minimum Value property set. |
| Range must be <= {maximum value} | This annotation displays on any Items with a Maximum Value property set. |
| Verbatim for Coding (Dictionary = {Dictionary Name}) | This annotation displays on any Item used as a Verbatim for coding. |
| Defaulted value selected for the Item: {Defaulted Value} | This annotation displays on Imaging items. |
Export Annotations
This option adds an annotation to each Item in the PDF that indicates where that Item is included in the Main Study Extract CSV. For example, the Date of Birth item may have an annotation Demographics.DOB. In this example, the item is in the Demographics form’s view, in a column labeled “DOB”.
Export annotations are only available in vaults where the View Sets feature is enabled. Contact your Veeva Services representative for details.
Indent Progressive Display Items
Selecting Indent Progressive Display Items indents Items configured for progressive display and displays the dependent Items directly below the controlling Item. Learn more about progressive display.
If you don’t select this checkbox, Vault doesn’t include any indication that an Item is configured for progressive display in the PDF.
Vault can only show 10 levels of progressive display dependency in the PDF. If you have more than 10, Vault stops indenting all items after the 10th level.
Study Design Specification (SDS)
The Study Design Specification (SDS) is a Microsoft Excel™ workbook. That workbook contains worksheets for key study design components:
- Schedule - Grid: Lists all Form Definitions within the Study in the order that they appear in the study schedule.
- Schedule - Tree: Lists all Event Group Definitions, Event Definitions, and Form Definitions, grouped together in the order that they appear in the study schedule.
- Form Definitions: Lists each Form Definition within the Study, with columns for all form-related properties.
- Assessments: Lists type and status of Assessments that have been performed within a Study.
- Codelists: Lists each Codelist Definition within the Study as a header row, with rows for each Codelist Item Definition for a codelist.
- Unit Codelists: Lists each Unit Definition within the Study as a header row, with rows for each Unit Item Definition for a unit.
- Review Plans: Lists Review Plan descriptions, types, and event dates.
- Rules (Optional): Lists each Rule Definition within the Study, with columns for rule details (such as the rule expression) and properties.
- Casebook Variables: Lists each Casebook Variable within the Study with columns for variable details and the variable’s mapped Item or Event Date.
- Study Settings: Lists the configured settings for the Study, including the Standard Date Format, the Subject ID Gen Format, and more.
- Subject Groups: Lists all Subject Groups within the Study, including the Label, Code, Type and External ID.
- Medical Coding Items (in vaults where Vault Coder is enabled): Lists all configured Medical Coding Item Definitions within the Study.
- Primary Export View (Optional): Lists all columns in the Study Data Extract view set for the Study.
- Protocol Deviations (in Studies where the Protocol Deviations feature is enabled): Lists all configured Categories, Subcategories, and Severities for protocol deviations within the Study.
- Local Labs: Lists Local Lab configuration settings including System General Settings, Study General Settings, and Study Setting Clinical Significance.
- Classifications: Lists all Classifications and their Values, and whether they are Active (Enabled).
- Summary: A summary sheet that includes the Vault name, Study name, Casebook Version number, Study Build number, description, Casebook Definition name, Change Reason, External ID, and document number.
- Repeating Event Groups: Lists all repeating Event Group Definitions within the Study, with the Event Group Name, Sequence, Default Display, Offset Days, Day Range Early, and Day Range Late for each event group.
- Integration Configuration: When enabled, lists all Integration Configurations (procedures).
- Data Loader Configurations (When Enabled): Lists all Data Loader Configurations for your Study, including column mappings
- Safety Settings: Lists values for Safety Settings for your Study. If your study isn’t using the safety integration, this won’t show in the SDS.
- Safety Form Configurations: Lists Safety Form Configurations for your Study. If your study isn’t using the safety integration, this won’t show in the SDS.
The Schedule - Tree and Form Definitions sheets include a Last Modified Date column, which displays the last date the object was updated, and a Relationship Last Modified Date column, which displays the date the child object was last associated with the parent object (for example, the date the Event was last associated with its parent Event Group).
SDS Options
You can choose to Include Rules and Include Export (if the View Sets feature is enabled in your vault) during SDS generation. This adds the Rules (for including rules) and Primary Export View (for including export) sheets to your Excel™ file.
You can also choose to include Keys and Origin details.
- Include Keys: This option adds columns for Definition Cross Vault Unique ID and DefinitionPrivate Key. The Cross Vault Unique ID provides the vault, study, and record IDs and is unique to the version of the object. The Private Key is used to define the object across versions and studies.
- Include Origin: This adds columns for origin keys. This set of keys tie the object to its point of origin, typically the library. These include the Origin Name (name of the study or library collection), Origin Type (either Study or Library), the Origin Definition Name (the current Name of the object in the origin study or library), and the Origin Key (the Cross Vault Unique ID tying the object to the Origin).
Vault includes these additional columns on the Schedule - Tree, Form Definitions, Rules, Codelists, and Units worksheets.
How to Create Study Specifications
To create study specifications:
- Navigate to your Study in Studio.
- Optional: To generate a specification for an older casebook version, select that version from the Actions menu.
-
From the Actions menu, click Create Specifications. The Create Specification dialog opens.
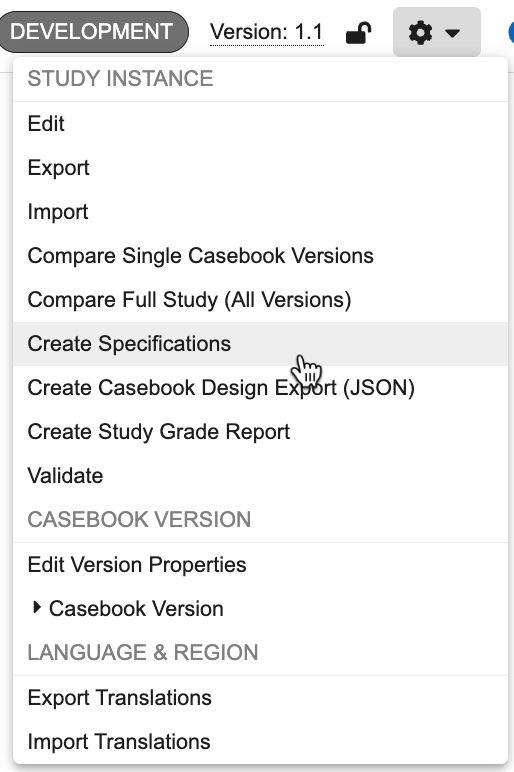
-
In the Create Specification dialog, select the types of documentation to generate (All CRF PDF, Unique CRF PDF, Include SDS).
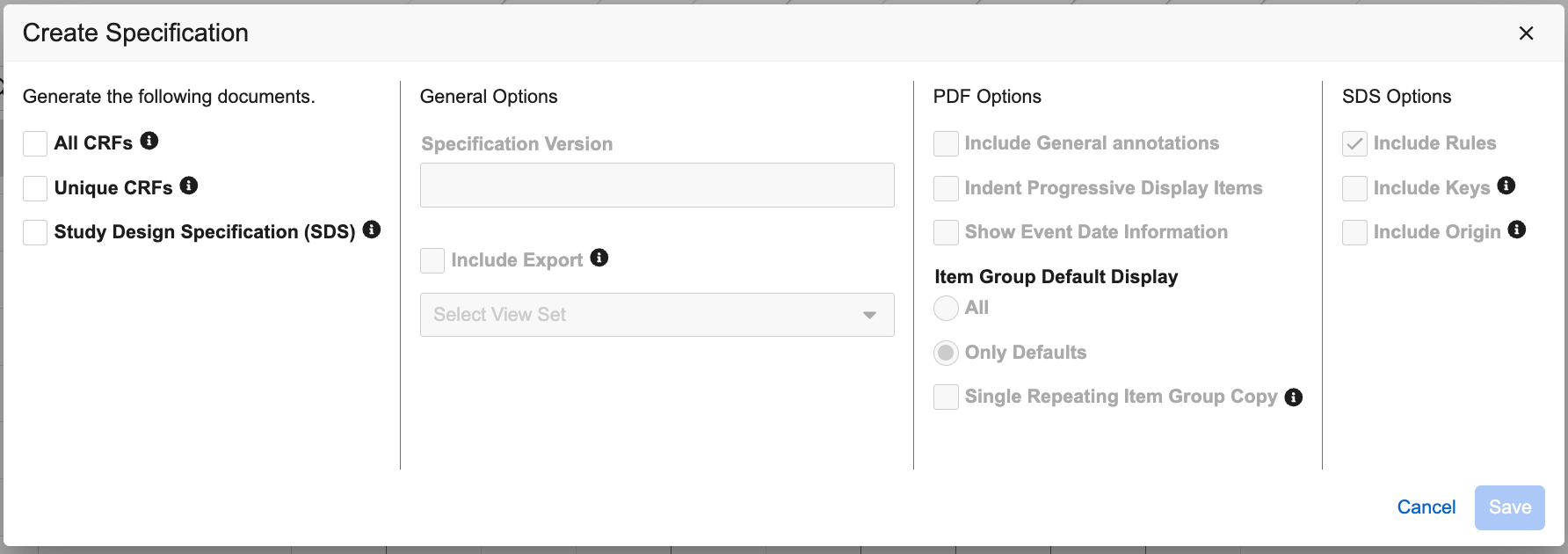
- Optional: Enter the Specification Version.
- Optional: Choose whether to Include Export viewsets.
- Optional: Select whether to Include Form Names on Forms and Bookmarks. When this option is selected, Form names will appear in parentheses next to the form labels within the PDFs and Bookmarks.
- Optional: Select whether to Include General Annotations.
- Optional: Select whether to Indent Progressive Display Items.
- Optional: Select whether to Show Event Date Information.
-
Optional: For Item Group Default Display, choose whether to display All data or Only Defaults.

- Optional: Select Single Repeating Item Group Copy to create a single copy of the repeating Item Group. When unchecked, this will create extra copies of repeating Item Groups in the PDFs.
- Optional: Select Add Schedule Driven Default Appendix to include an appendix at the end of the PDF for schedule driven defaults that are used in the Form design.
- Click Save.
- Vault begins a job to generate your study specifications. When finished, Vault sends you a notification with a link to download the file.
Configuring Item Group Default Display in PDFs
You can configure how default item groups are displayed in PDFs through the Item Group Default Display options in the Create Specification dialog.
The following table details how default data is presented in the output PDF when different options are selected.
Casebook Design Export
The CDE, or Casebook Design Export, is a JSON representation of a Study’s design at a specific Casebook Version. You can retrieve the CDE via the EDC API or in Studio.
To generate the CDE in Studio:
- Navigate to your Study in Studio.
-
Select Create Casebook Design Export (JSON) from the Actions menu.
- Click Create in the Create Casebook Design Export dialog.
- Vault begins a job to generate the CDE. When finished, Vault sends you a notification with a link to download the file.
Related Permissions
Users with the standard CDMS Study Designer study role can perform the actions described here by default.