OpenEDC: Third Party EDC Studies
CDB supports the use of CDB for data from third party EDC applications, without data collection or study design occurring in Veeva EDC. CDB still uses Veeva EDC for user management.
Prerequisites
Contact your Veeva representative to enable the OpenEDC feature for your vault.
You must create a Study record in Veeva EDC, using the External study type. Then, your organization must create users and assign them roles from within Clinical Data to allow them access to CDB.
Users with the CDMS Lead Data Manager or CDMS Super User standard study roles can perform the actions described below by default. If your organization uses custom roles, your role must grant the following permissions:
| Type | Permission Label | Controls |
|---|---|---|
| Standard Tab | Workbench Tab | Ability to access and use the Data Workbench application, via the Workbench tab |
| Functional Permission | API Access | Ability to access and use the Vault EDC API. (This permission is also required to use CDB.) |
| Functional Permission | View Import | Ability to access the Import page |
| Functional Permission | Approve Import | Ability to approve or reject an import package that contains configuration changes |
| Functional Permission | Download Import Package | Ability to download import packages |
| Functional Permission | Manage Sources | Ability to view and manage Sources from the import of third party data in CDB |
If your Study contains restricted data, you must have the Restricted Data Access permission to view it.
Learn more about Study Roles.
Configuration in Veeva EDC
You must first create your Study record in EDC Studio. The creation of an external EDC study in Studio creates the Study on the EDC side, so that you can use Clinical Data’s System Tools to manage permissions and data access. CDB makes API calls to check that your Study exists and to check if a user has permission to a Study as needed. There is no information that is sent from Veeva EDC to CDB.
- Learn how to create a Study with the External type.
- Learn how to manage user access.
Sites & Subjects
CDB uses the Site and Subject columns in your import CSVs to create Sites. Because CDB only has the data in these files to create Sites from, only @HDR.Site.Name and @HDR.Subject.Name are available for @HDR context.
How to Import
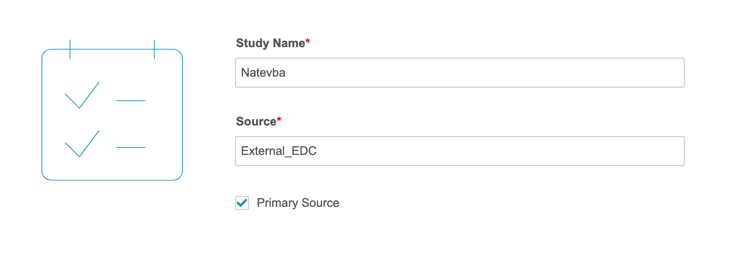
Importing data for OpenEDC works in the same way as importing any third party data into CDB. You will need to specify that you’re using OpenEDC with the “primary_source” property in your manifest.json file. Setting “primary_source” to true indicates that the import package is the primary data source for the Study.
Use the following in your manifest.json file:
{
"study": "studyName",
"source": "External_EDC",
"primary_source": true,
}
To do this in the CDB Manifest Builder, select the Primary Source checkbox.
Veeva EDC always acts as the Primary Source for its studies. If you try to import a 3PD package as the Primary Source for an EDC study, the system blocks the import and generates a P-030 error.:
Learn how to:
What Isn’t Included
In the current release, the following functionality isn’t supported in OpenEDC studies:
- Unit Codelists: CDB doesn’t support Unit Codelists. If you want to show coded and decoded unit values, you will need to include them in separate data columns. Non-unit codelists are supported.
- Labels: The import of Labels is only supported for Forms and Items. If you want to see Labels for an Event or Item Group, you will need to include them in separate data columns.
- Key Mappings: CDB doesn’t support the import of key mappings for the primary source in an OpenEDC study. You can use key mappings for third party data sources in an OpenEDC study.
- Protocol Deviations: CDB doesn’t support the creation of Protocol Deviations in OpenEDC studies. This feature requires study design configuration in Veeva EDC.
- Clean Patient Tracker: CDB doesn’t offer the Clean Patient Tracker in OpenEDC studies.
- Study File Format (SFF) API: SFF does not support OpenEDC studies. It does support third party studies with existing EDC data.